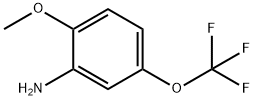
2-METHOXY-5-(TRIFLUOROMETHOXY)ANILINE synthesis
- Product Name:2-METHOXY-5-(TRIFLUOROMETHOXY)ANILINE
- CAS Number:660848-57-7
- Molecular formula:C8H8F3NO2
- Molecular Weight:207.15
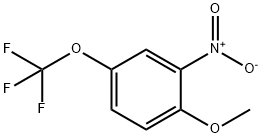
660848-54-4
10 suppliers
inquiry

660848-57-7
51 suppliers
$22.00/100mg
Yield:660848-57-7 81%
Reaction Conditions:
with sodium dithionite;water in tetrahydrofuran at 20; for 16 h;
Steps:
21
Synthesis of Compound S21-3.; To a solution of compound S21-2 (0.10 mol, 1 equiv) in THF (600 mL) at 0 °C was added a solution of Na2S204 (102.4 g, 85%, 0.50 mol, 5 equiv) in water (400 mL). The reaction was stirred at rt for 16 hrs. The organic layer was collected. The aqueous later was extracted with EtOAc (100 mL x 3). The combined organic layers were dried over sodium sulfate and concentrated. EtOAc (200 mL) was added to the residue. The insoluble material was filtered. The filtrate was collected. Aqueous HCl (150 mL, 2 N) and methanol (150 mL) were added to the solid. The mixture was stirred at rt for 2 hrs, neutralized with aqueous NaOH (6 N), and extracted with EtOAc (100 mL x 3). The extracts were combined with the previously saved EtOAc filtrate, dried over sodium sulfate, and concentrated to dryness to yield the desired product S21-3 as a deep yellow liquid (16.69 g, 81%): Rf = 0.50 (20%EtOAc/hexane); 1H NMR (400 MHz, CDC13) δ 6.70 (d, J= 9.2 Hz, 1 H), 6.59 (s, 1 H), 6.57 (d, J= 9.2 Hz, 1 H), 3.83 (s, 3 H); MS (ESI) m/z 208.0 (M+H).
References:
WO2012/21712,2012,A1 Location in patent:Page/Page column 232
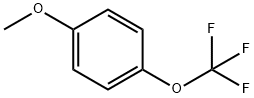
710-18-9
174 suppliers
$5.00/1g

660848-57-7
51 suppliers
$22.00/100mg