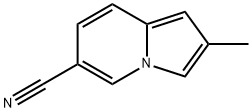
2-Methyl-6-indolizinecarbonitrile synthesis
- Product Name:2-Methyl-6-indolizinecarbonitrile
- CAS Number:22320-36-1
- Molecular formula:C10H8N2
- Molecular Weight:156.18
Yield:22320-36-1 84%
Reaction Conditions:
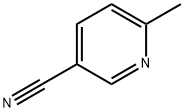
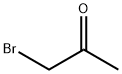
Stage #1: 6-methylnicotinonitrile;1-bromoacetone in sulfolane at 45; for 48 h;
Stage #2: with sodium hydrogencarbonate in water; for 1 h;Heating / reflux;
Steps:
24
1-Bromo-propan-2-one (2.26 ml) is added under an atmosphere of argon to a solution of 5- cyano-2-methylpyridine (5.0 g, 41.9 mmol) in sulfolane (30 ml). The reaction mixture is stirred for 48 hours at 45 0C, then diluted with EtOAc. The precipitated salt is filtered off and dissolved in water (50 ml). The aqueous solution is washed with EtOAc, then a 10% aqueous solution of NaHCO3 (30 ml) is added, and the mixture is heated to reflux for 1 hour. After cooling, the reaction mixture is filtered, and the filtrate is extracted three times with EtOAc. The combined organic layers are dried over Na2SO4, filtered, and concentrated in vacuo. The residue is purified via flash chromatography (hexane / EtOAc 8 : 1 , + 0.2% NEt3) to afford the title compound (5.50 g, 84%). 1H NMR (400 MHz, d6-DMSO): δ = 9.10 - 9.09 (m, 1 H), 7.59 (s, 1 H), 7.55 (d, J = 9.0 Hz, 1 H), 6.90 (dd, J = 9.3 / 1.7 Hz, 1 H), 6.55 (s, 1 H), 2.39 (s, 3H). MS (ES+): 157 (M+H)+.
References:
WO2008/74752,2008,A2 Location in patent:Page/Page column 20-21