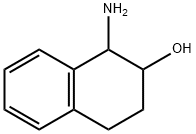
2-Naphthalenol, 1-amino-1,2,3,4-tetrahydro- synthesis
- Product Name:2-Naphthalenol, 1-amino-1,2,3,4-tetrahydro-
- CAS Number:90874-85-4
- Molecular formula:C10H13NO
- Molecular Weight:163.22
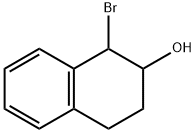
1195628-70-6
3 suppliers
inquiry

90874-85-4
11 suppliers
inquiry
Yield:90874-85-4 76%
Reaction Conditions:
with ammonium hydroxide in methanol;water at 20;
Steps:
1-amino-1,2,3,4-tetrahydronaphthalen-2-ol (106)
Compound 105 (770 mg, 3.4 mmol) was dissolved in 2 mL of meOH after which aqueous ammonium hydroxide (4 mL, 29 mmol) was added. The mixture was stirred overnight at ambient temperature. TLC verified disappearance of the starting product and the meOH was evaporated. The residue was purified directly by silica gel chromatography (gradient system 0 to 10% meOH/100 to 90% ch2Cl2). Product 106 was obtained in the form of a white solid (423 mg, 76%). R (aE 100): 0.19. 1H NMR (400 MHz, dMSO-d6) δ 7.52 (m, 1H), 7.24 (m, 2H), 7.16 (m, 1H), 5.46 (br s, 1H, OH), 4.02 (m, 1H), 3.80 (m, 1H), 2.81 (m, 2H), 2.02 (m, 1H), 1.75 (m, 1H). 13C NMR (101 MHz, dMSO-d6) δ 136.6, 132.9, 128.7, 128.0, 127.7, 126.1, 68.5, 55.3, 28.2, 26.3. IR (diamond aTR, cm-1) ν 2929, 1598, 1493, 1043, 774, 742, 552. HRMS (EI-MS): m/z calculated for C10H14NO [M+H]+: 164.1069, found: 164.1070. Tm: 155-160° C.
References:
US2021/9581,2021,A1 Location in patent:Paragraph 0280; 0281
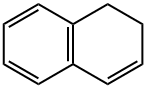
447-53-0
114 suppliers
$12.00/1g

90874-85-4
11 suppliers
inquiry

103028-83-7
20 suppliers
inquiry

90874-85-4
11 suppliers
inquiry
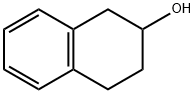
530-91-6
89 suppliers
$15.00/100mg

90874-85-4
11 suppliers
inquiry

64245-04-1
4 suppliers
inquiry

90874-85-4
11 suppliers
inquiry