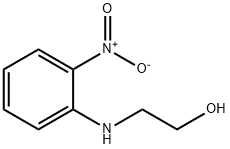
2-Nitro-N-hydroxyethyl aniline synthesis
- Product Name:2-Nitro-N-hydroxyethyl aniline
- CAS Number:4926-55-0
- Molecular formula:C8H10N2O3
- Molecular Weight:182.18
Yield:-
Reaction Conditions:
with hydrogenchloride;potassium hydroxide;sulfuric acid;calcium carbonate in anhydrous ethylene glycol dimethyl ether;water
Steps:
1 Preparation of 2-hydroxyethylamino-nitrobenzene
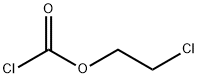
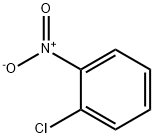
EXAMPLE 1 Preparation of 2-hydroxyethylamino-nitrobenzene 60.1 g of calcium carbonate are added to a solution of 138.1 g of 2-nitroaniline in 360 g of monoethylene glycol dimethylether and 60 g of water. 150.0 g of 2-chloroethyl chloroformate are then metered in at 80° C. and the reaction is brought to completion by subsequent stirring at 80° C. 100 g of water and 8.7 g of 35% strength hydrochloric acid are then added, up to a pH of 1, and the lower aqueous phase is separated off. The upper organic phase is diluted with 90 g of monoethylene glycol dimethylether, and 370.6 g of a 50% strength potassium hydroxide solution are added at 15° C. The reaction mixture is then heated to 85° C. and subsequently stirred at 85° C. for 4 hours. Thereafter, 250 g of water are added and the lower salt-containing aqueous phase is separated off. First 500 g of water and then 9 g of 10% strength sulphuric acid are added to the yellow-red organic phase, up to a pH of 8.5. After the monoethylene glycol dimethyl ether has been distilled off and the residue has been cooled to 10° C., the product is obtained in the form of yellow-red crystals. Yield: 173.4 g Purity: 99.8%
References:
Cassella Aktiengesellschaft US5508467, 1996, A

141-43-5
853 suppliers
$9.00/10g
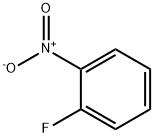
1493-27-2
498 suppliers
$5.00/10g

4926-55-0
175 suppliers
$7.00/10g
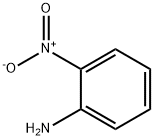
88-74-4
8 suppliers
$10.00/1g

4926-55-0
175 suppliers
$7.00/10g