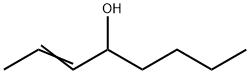
2-OCTEN-4-OL synthesis
- Product Name:2-OCTEN-4-OL
- CAS Number:4798-61-2
- Molecular formula:C8H16O
- Molecular Weight:128.21
22286-99-3
0 suppliers
inquiry

4798-61-2
13 suppliers
inquiry
Yield:4798-61-2 90%
Reaction Conditions:
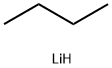
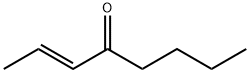
Stage #1: (E)-2-octen-4-onewith lithium aluminium tetrahydride in tetrahydrofuran at 5 - 20; for 1.5 h;Inert atmosphere;
Stage #2: with water;sodium hydroxide in tetrahydrofuran at 7; for 1 h;Inert atmosphere;
Steps:
1.b (b) Synthesis of (E)-oct-2-en-4-yl 2-oxo-2-phenylacetate (Compound O2)
Under nitrogen a dispersion of LiAlhU (1.8 g, 47.4 mmol) in tetrahydrofuran (THF, 50 ml_) was cooled on an ice bath. (E)-Oct-2-en-4-one (10.0 g, 79.2 mmol) in THF (50 ml_) was added dropwise during 30 min while keeping the reaction temperature below 5°C. After stirring at room temperature for 1 h, the mixture was cooled at 0°C with an ice bath and water (1.8 g) was added very slowly while keeping the reaction temperature below 7°C. Then an aqueous solution of NaOH (10%, 1.8 g) and water (5.4 g) were added. The ice bath was removed and the mixture was stirred for 1 h. A white precipitate was slowly formed. Sodium sulphate (10.0 g) was added and the reaction mixture filtered. THF was removed under reduced pressure (40°C, 4 mbar, 2 h) to give 9.59 g (90%) of (E)-oct-2-en-4-ol.1H-NMR: 5.70-5.60 (m, 1 H), 5.52-5.44 (m, 1 H), 4.02 (q, J = 6.7, 1 H), 1.70 (dd, J = 6.4, 1.3, 3 H), 1.63-1.41 (m, 3 H), 1.39-1.23 (m, 4 H), 0.90 (t, J = 7.1 , 3 H). 13C-NMR: 134.46, 126.68, 73.17, 37.03, 27.69, 22.66, 17.68, 14.07.
References:
WO2021/214205,2021,A1 Location in patent:Page/Page column 24; 25