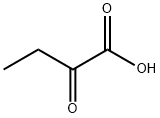
2-Oxobutyric acid synthesis
- Product Name:2-Oxobutyric acid
- CAS Number:600-18-0
- Molecular formula:C4H6O3
- Molecular Weight:102.09
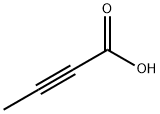
590-93-2
309 suppliers
$6.00/1g

600-18-0
244 suppliers
$23.00/1g
Yield:600-18-0 35%
Reaction Conditions:
with water;ruthenium trichloride; pH=1.3 - 2.0 at 80 - 100; for 12 - 36 h;Product distribution / selectivity;
Steps:
9; 18; 24; 40; 41
[Example 9]; 0.1 mmol of water-soluble acetylene carboxylic acids whose R1 is CH3 and R2 is H in Fig. 1(a) was mixed with 2 mL of aqueous solution containing 5.0 µmol of ruthenium trichloride, and the mixture was reacted at the pH 2.0 at 80°C under argon atmosphere for 12 hours. The resulting mixture was condensed to give a product. The product was analyzed by 1H NMR.; [Example 24]; 0.1 mmol of water-soluble acetylene dicarboxylic acids whose R1 is CH3 and R2 is H in Fig. 1(a) was mixed with 2 mL of aqueous solution containing 1.0 µmol of ruthenium trichloride, and the mixture was reacted at the pH 1.3 at 100°C under argon atmosphere for 24 hours. The resulting reaction mixture was condensed to give a product. The product was analyzed by 1H NMR.; [Example 18]; 5 mmol of water-soluble acetylene-carboxylic acids whose R1 is CH3 and R2 is H in Fig. 3 was poured into 10 mL of water so as to have the pH 2.0. 5.0 µmol of ruthenium chloride was added to the solution, and the resulting mixture was reacted at 80°C under argon atmosphere. After 36 hours, 10 mmol of formate ammonium and 5.0 µmol of aqua(η5-tetramethylcyclopentadienyl) rhodium (III)(2,2'-bipyridyl) sulphate were added, and the resulting mixture was further reacted for two hours. After completion of the reaction, the resulting product was analyzed by 1H NMR.; [Example 19]; 5 mmol of water-soluble acetylene-carboxylic acids whose R1 is CH3 and R2 is H in Fig. 3 was poured into 10 mL of water so as to have the pH 2.0. 5.0 µmol of ruthenium chloride was added to the solution, and the resulting mixture was reacted at 80°C under argon atmosphere. After 36 hours, 10 mmol of formate ammonium and 5.0 µmol of (η5-tetramethylcyclopentadienyl) rhodium (III)(2,2'-bipyridyl) aqua complex were added, and the resulting mixture was further reacted for two hours. After completion of the reaction, the resulting product was analyzed by 1H NMR.; [Example 40]; 0.1 mmol of water-soluble acetylene-carboxylic acids whose R1 is CH3 and R2 is H in Fig. 3 was poured into 2 mL of water so as to have the pH 1.3. 1.0 µmol of ruthenium chloride was added to the solution, and the resulting mixture was reacted at 100°C under argon atmosphere. After 12 hours, 4 mmol of formate ammonium and 1.0 µmol of aqua(n5-tetramethylcyclopentadienyl) rhodium (III)(2,2'-bipyridyl) sulphate were added, and the resulting mixture was further reacted at 80°C for an hour. After completion of the reaction, the resulting product was analyzed by 1H NMR.; [Example 41]; 0.1 mmol of water-soluble acetylene-carboxylic acids whose R1 is CH3 and R2 is H in Fig. 3 was poured into 2 mL of water so as to have the pH 1.3. 1.0 µmol of ruthenium chloride was added to the solution, and the resulting mixture was reacted at 100°C under argon atmosphere. After 12 hours, 4 mmol of formate ammonium and 1.0 µmol of aqua(η5-tetramethylcyclopentadienyl) iridium (III)(2,2'-bipyridyl) sulphate were added, and the resulting mixture was further reacted at 80°C for an hour. After completion of the reaction, the resulting product was analyzed by 1H NMR.
References:
Japan Science and Technology Agency EP1932824, 2008, A1 Location in patent:Page/Page column 18; 19; 20; 21; 25; 26-27
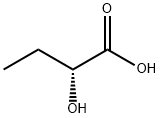
20016-85-7
52 suppliers
$42.00/100mg

600-18-0
244 suppliers
$23.00/1g
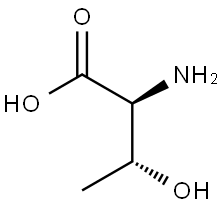
72-19-5
668 suppliers
$4.76/25g

600-18-0
244 suppliers
$23.00/1g
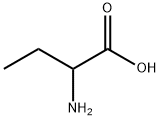
2835-81-6
360 suppliers
$5.00/5g

600-18-0
244 suppliers
$23.00/1g
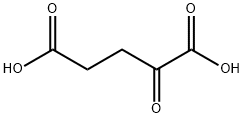
328-50-7
542 suppliers
$6.00/25g
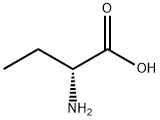
2623-91-8
369 suppliers
$5.00/5g

600-18-0
244 suppliers
$23.00/1g
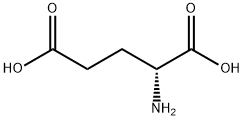
6893-26-1
457 suppliers
$6.00/10g