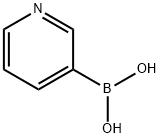
2-Pyridineboronic acid synthesis
- Product Name:2-Pyridineboronic acid
- CAS Number:197958-29-5
- Molecular formula:C5H6BNO2
- Molecular Weight:122.92
109-04-6
519 suppliers
$6.00/25g

5419-55-6
362 suppliers
$12.00/5g

197958-29-5
200 suppliers
$12.00/250mg
Yield:197958-29-5 81%
Reaction Conditions:
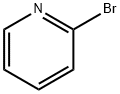
Stage #1:2-bromo-pyridine;Triisopropyl borate with n-butyllithium in tetrahydrofuran;hexane;toluene at -30; for 3 h;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;hexane;water;toluene at 20; for 1 h;Inert atmosphere;
Steps:
1.2 Step 2. Synthesis of Compound VI-1
1.58 g (10 mmol) of compound V-1 and 3.76 g (20 mmol) of B(i-PrO)3 were dissolved in 14 mL of dry toluene and 7 mL of THF, stirred and cooled to -30°C under a nitrogen atmosphere, and then slowly dried with a syringe. A solution of 6.25 mL (10 mmol) of 1.6 M n-BuLi in n-hexane was added dropwise. After completion of the addition, the reaction mixture was stirred at this temperature for 3 hours and then at room temperature for a further 3 hours, and the reaction was completed by TLC. To the reaction mixture was slowly added 1 mL of concentrated hydrochloric acid, stirred at room temperature for 1 hour, and poured into 200 mL of ice water.Where, pH=8 was adjusted with saturated NaHCO 3 solution, stirred, extracted with 50 mL×3 CH 2 Cl 2 , the combined extracts were washed with 100 mL 5% brine and dried over anhydrous sodium sulfate. The desiccant was filtered off with suction, the filtrate was evaporated to dryness on a rotary evaporator and the residue was purified using silica gel column chromatography to afford compound VI-I, 1.00 g (81% yield).
References:
Foshan Sai Weisi Pharmaceutical Technology Co., Ltd.;Cai Ziyang CN107903250, 2018, A Location in patent:Paragraph 0025; 0029; 0030; 0031

109-04-6
519 suppliers
$6.00/25g

121-43-7
342 suppliers
$13.00/25mL

197958-29-5
200 suppliers
$12.00/250mg

109-04-6
519 suppliers
$6.00/25g

197958-29-5
200 suppliers
$12.00/250mg