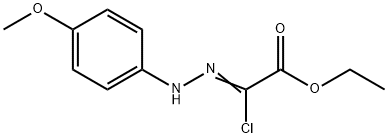
Acetic acid, 2-chloro-2-[2-(4-methoxyphenyl)hydrazinylidene], ethyl ester synthesis
- Product Name:Acetic acid, 2-chloro-2-[2-(4-methoxyphenyl)hydrazinylidene], ethyl ester
- CAS Number:27143-07-3
- Molecular formula:C11H13ClN2O3
- Molecular Weight:256.69
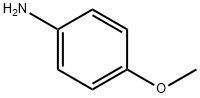
104-94-9
473 suppliers
$9.00/1g
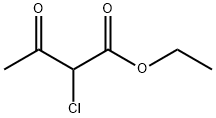
609-15-4
437 suppliers
$6.00/25g
![Acetic acid, 2-chloro-2-[2-(4-methoxyphenyl)hydrazinylidene], ethyl ester](/CAS2/GIF/27143-07-3.gif)
27143-07-3
453 suppliers
$6.00/250mg
Yield:27143-07-3 89%
Reaction Conditions:
Stage #1: 4-methoxy-anilinewith hydrogenchloride;sodium nitrite in water at -10 - -5; for 0.5 h;
Stage #2: ethyl 2-chloro-3-oxo-butyratewith sodium acetate in water at -10 - -5; for 2 h;
Steps:
1.3
100 g (0.81 mol) of the starting material (3) was added to 400 ml of water at room temperature,To the reaction solution was added dropwise 270 ml (3.24 mol) of concentrated hydrochloric acid.After the dropwise addition, 115 g (1.62O1) of a mixed solution of sodium nitrite and 460 ml of water was added dropwise to the reaction solution, and the temperature control was carried out at -5 to 10C.Drop control, control the temperature at -5 ~ -10 ° C reaction 30min.Then, 135 ml (0.97 mol 1) of the reaction solution was added dropwise to the reaction solution,A mixed solution of ethyl dichloroacetoacetate and 135 ml of methanol,The temperature during the dropwise process is controlled at -5 to 10 ° C.After completion of the reaction, a mixed solution of 207 g (2.43 mol) of anhydrous sodium acetate and 514 ml of water was added dropwise to the reaction solution,The temperature is maintained at -5 to 10 ° C during the dropwise addition. Drop finished, temperature control at _10 ° C, reaction 2h.Then transferred to room temperature, allowed to stand overnight, with a brown solid precipitated, filtered and the filter cake was washed with anhydrous methanol (2 x 300ml).To give 186.9 g of a yellow solid (theoretical yield of 208. lg) in a yield of 89%
References:
CN104513239,2017,B Location in patent:Paragraph 0197; 0238; 0243; 0244
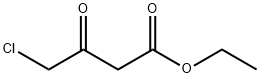
638-07-3
465 suppliers
$5.00/5G

104-94-9
473 suppliers
$9.00/1g
![Acetic acid, 2-chloro-2-[2-(4-methoxyphenyl)hydrazinylidene], ethyl ester](/CAS2/GIF/27143-07-3.gif)
27143-07-3
453 suppliers
$6.00/250mg

104-94-9
473 suppliers
$9.00/1g
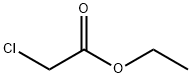
105-39-5
380 suppliers
$10.00/5g
![Acetic acid, 2-chloro-2-[2-(4-methoxyphenyl)hydrazinylidene], ethyl ester](/CAS2/GIF/27143-07-3.gif)
27143-07-3
453 suppliers
$6.00/250mg