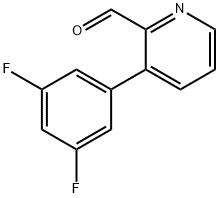
3-(3,5-DIFLUOROPHENYL)PICOLINALDEHYDE synthesis
- Product Name:3-(3,5-DIFLUOROPHENYL)PICOLINALDEHYDE
- CAS Number:780801-43-6
- Molecular formula:C12H7F2NO
- Molecular Weight:219.19
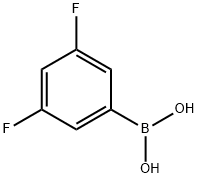
156545-07-2
401 suppliers
$5.00/1g
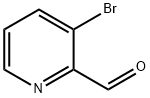
405174-97-2
148 suppliers
$19.00/100mg

780801-43-6
6 suppliers
inquiry
Yield:780801-43-6 61%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in tetrahydrofuran;1,2-dimethoxyethane at 90; for 16 h;
Steps:
67 EXAMPLE 67, COMPOUND 67: N1-[3-(3,5-Difluoro-phenyl)-pyridin-2-ylmethyl]-N1-(3,5-dimethyl-pyridin-2-ylmethyl)-butane-1,4-diamine HCl salt
EXAMPLE 67 COMPOUND 67: N1-[3-(3,5-Difluoro-phenyl)-pyridin-2-ylmethyl]-N1-(3,5-dimethyl-pyridin-2-ylmethyl)-butane-1,4-diamine HCl salt To a solution of 3-bromo-pyridine-2-carbaldehyde (1.2 g, 6.45 mmol) dissolved in ethylene glycol dimethyl ether (25 mL), THF(10 mL) and saturated solution of Na2CO3 (9 mL) was added 3,5 difluorophenyl boronic acid (1.12 g, 7.10 mmol). Purge the mixture with Ar gas (10 min). To this mixture was added Pd(PPh3)4 (373 mg, 0.33 mmol) and stir under a positive pressure of Ar at 90° C. for 16 hours. The reaction mixture was quenched with a solution of saturated NaHCO3 (50 mL). Extract with CH2Cl2 (2*50 mL). The combined organic extracts were dried (Na2SO4), filtered, and concentrated in vacuo to afford a light yellow oil. Purification via column chromatography on silica gel (CH2Cl2:MeOH: 80:20, v/v/v) afforded 3-(3,5-difluoro-phenyl)-pyridine-2-carbaldehyde as a white solid (0.86 g, 61%). 1H NMR (CDCl3) δ 6.59 (m, 1H), 6.92 (m, 2H), 7.60 (m, 1H), 7.76 (d, 1H, J=7.5 Hz), 8.88 (d, 1H, J=3.5 Hz), 10.11 (s, 1H).
References:
US2004/209921,2004,A1 Location in patent:Page 41