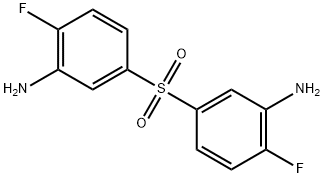
3,3'-Sulfonylbis[6-fluoroaniline] synthesis
- Product Name:3,3'-Sulfonylbis[6-fluoroaniline]
- CAS Number:40939-65-9
- Molecular formula:C12H10F2N2O2S
- Molecular Weight:284.28
312-30-1
73 suppliers
$30.00/1g
![3,3'-Sulfonylbis[6-fluoroaniline]](/CAS2/GIF/40939-65-9.gif)
40939-65-9
12 suppliers
inquiry
Yield:40939-65-9 90%
Reaction Conditions:
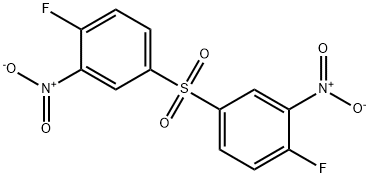
Stage #1: 3,3'-dinitro-4,4'-difluorodiphenyl sulphonewith sulfuric acid in water at 100; pH=4; for 3.33333 h;
Stage #2: in water; pH=7; for 0.25 h;Product distribution / selectivity;
Steps:
Fourth Recycle (R4): In the same set up as described above, 500 ml water, heated to 100°C. Charged 20% H2S04 to get pH 4.0 and 50 g NRC start up with continuous stirring at 100°C. First lot of lOg NRC and first lot of 11.2 g Nitro was charged to the reaction mass in 20 min. The reaction mass was maintained for 20 min at 100°C. Remaining NRC & Nitro was charged in four equal lots in similar manner as followed for first lot. NGC was charged to adjust pH of reaction mass to 7.0 and maintain for 15 minutes. Then filter whole batch take completely dry grinded spent charged 150ml DMF, heated to 80°c. Stir it for 2hr then it is decanted and collected in separate flask for further processing. Charged 150 ml DMF was charged for extraction, stir it for 2hr at 80°C and stirring was stopped and upper liquid layer was decanted to the crystallizer. Again 150 ml DMF was added to solid inorganic by-product, remaining in the flask under stirring at 80°C for 2hr.Then filter it give wash of 25 ml hot DMF. Collect all decanted mass & washing. Distill out approx. 60-70% from total volume of DMF at 80-85°c in vacuum distillation. Chill distillate up to 0-5°c, crystalline material was filtered, to get on drying 90 g Off white powder with purity 99.39%. Mother liquor was recycled in subsequent batches.
References:
WO2011/48535,2011,A1 Location in patent:Page/Page column 51; 53-54