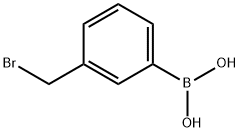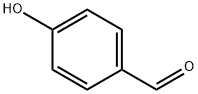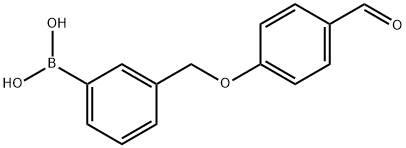
3-((4-formylphenoxy)methyl)phenylboronic acid synthesis
- Product Name:3-((4-formylphenoxy)methyl)phenylboronic acid
- CAS Number:1229652-27-0
- Molecular formula:C14H13BO4
- Molecular Weight:256.06
Yield:1229652-27-0 24%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 12 h;
Steps:
4.1.1. General Procedure for the Preparation of Compounds 1a-18a
General procedure: A solution of 4-hydroxybenzaldehyde (1 g, 8.2 mmol), substituted benzyl bromide(8.4 mmol, 1.02 eq) and potassium carbonate (1.7 g, 12.3 mmol) in 30 mL of N,N-dimethylformamide (DMF) was stirred overnight under an inert atmosphere at room temperature[29]. Subsequently, 50 mL water was added and the mixture was extracted with ethylacetate (40 mL 3). The combined organic phase was washed with a saturated brine (30 mL 2), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressureto give the crude product, which was purified by column chromatography on silicagel (10-50% ethyl acetate in petroleum ether) to obtain the corresponding products, 1a-18a.(2-((4-Formylphenoxy)methyl)phenyl)boronic acid (1a).
References:
Jia, Ruifang;Zhang, Jiwei;Zhang, Jian;Bertagnin, Chiara;Bonomini, Anna;Guizzo, Laura;Gao, Zhen;Ji, Xiangkai;Li, Zhuo;Liu, Chuanfeng;Ju, Han;Ma, Xiuli;Loregian, Arianna;Huang, Bing;Zhan, Peng;Liu, Xinyong [Molecules,2022,vol. 27,# 19,art. no. 6426]