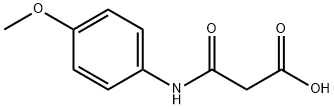
3-[(4-METHOXYPHENYL)AMINO]-3-OXOPROPANOIC ACID synthesis
- Product Name:3-[(4-METHOXYPHENYL)AMINO]-3-OXOPROPANOIC ACID
- CAS Number:61916-60-7
- Molecular formula:C10H11NO4
- Molecular Weight:209.2
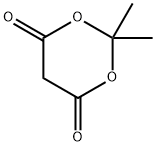
2033-24-1
627 suppliers
$6.00/25g
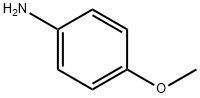
104-94-9
475 suppliers
$9.00/1g
![3-[(4-METHOXYPHENYL)AMINO]-3-OXOPROPANOIC ACID](/CAS/GIF/61916-60-7.gif)
61916-60-7
12 suppliers
$45.00/50mg
Yield:61916-60-7 77%
Reaction Conditions:
in neat (no solvent) at 60; for 4 h;
Steps:
Experimental details
General procedure: All reagents were obtained from commercial sources and used without further purification. Typical experimental procedures for the conversion of anilines 1 to malonic acid monoanilides 2 and from 2 to quinolinones 3 are described below using the syntheses 4-hydroxy-2-quinolinone (3a) as an example. A mixture of aniline (1a, 500 mg, 5.4 mmol) and Meldrum’s acid (774 mg, 5.4 mmol) in a test tube was heated for 4 hours at 60 C (reaction was complete as monitored by TLC). The reaction mixture was partitioned between aqueous sodium bicarbonate solution and ethylac etate. The aqueous layer was acidified with concentrated HCl to pH 1 and extracted with ethyl acetate (3 x 15 mL). The combined organic layer was dried over anhydrous sodium sulfate and the solvent was removed to yield malonic acid monoanilide 2a as a white solid (746 mg, 78%). A 25mL two neckedround-bottom flask was flame dried, charged with MSAA (587 mg, 3.4mmol), flushed with nitrogen gas, and fitted with a drying tube containing CaCl2. The flask was heated at 60 C until MSAA melted. Monoanilide 2a (300mg, 1.7 mmol) was added and the flask was flushed with nitrogen gas again. The reaction was complete after three hours (verified by TLC). The reaction was placed in a dry ice bath and triturated with ethyl acetate. The solid that formed was filtered and the solid was washed with cold water and ethyl acetate to produce quinolinone 3a as an off-white solid (244 mg, 90%).
References:
Amagata, Taro;Assad, Meerna Y.;Atalay, Sanberk S.;Wu, Weiming [Tetrahedron Letters,2020]
![Propanoic acid, 3-[(4-methoxyphenyl)amino]-3-oxo-, ethyl ester](/CAS/GIF/5382-15-0.gif)
5382-15-0
4 suppliers
inquiry
![3-[(4-METHOXYPHENYL)AMINO]-3-OXOPROPANOIC ACID](/CAS/GIF/61916-60-7.gif)
61916-60-7
12 suppliers
$45.00/50mg

104-94-9
475 suppliers
$9.00/1g
![3-[(4-METHOXYPHENYL)AMINO]-3-OXOPROPANOIC ACID](/CAS/GIF/61916-60-7.gif)
61916-60-7
12 suppliers
$45.00/50mg