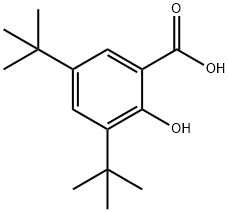
3,5-Bis-tert-butylsalicylic acid synthesis
- Product Name:3,5-Bis-tert-butylsalicylic acid
- CAS Number:19715-19-6
- Molecular formula:C15H22O3
- Molecular Weight:250.33
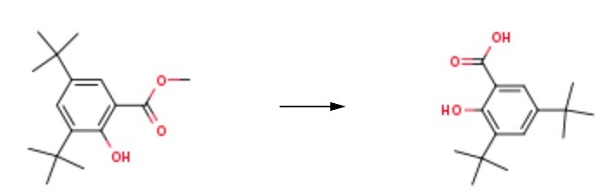

124-38-9
121 suppliers
$214.00/14L
96-76-4
358 suppliers
$6.00/25g

19715-19-6
197 suppliers
$8.00/5g
Yield:19715-19-6 98%
Reaction Conditions:
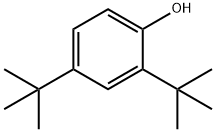
Stage #1: 2,4-di-tert-Butylphenolwith 1,3-dimethyl-2-imidazolidinone;sodium hydroxide in water;xylene;Heating / reflux;
Stage #2: carbon dioxide
Steps:
5 Example 5
From a mixture of a filtrate and a wash liquid in the step of the filtration of DBSA-Na obtained in a recovery yield of 90%/NaOH in Example 4, xylene was distilled off under heating to prepare a solution substantially comprising DBP, DMi and a solubility content of DBSA-Na (2 mol%/NaOH). Next, 10.03 g of DBP and 40 g of xylene were added to the solution, and the solution was heated until reflux began. While 4.06 g of a 49 wt % aqueous NaOH solution was added dropwise, azeotropic dehydration was carried out, and afterward, the same procedure as in Example 4 was conducted. As a result, DBSA having a purity of 99.8% was obtained in a yield of 98%/addition DBP. In the mixture of the filtrate and the wash liquid in the step of the filtration of DBSA-Na, 5 mol %/(produced DBSA-Na) of DBSA-Na was dissolved. The mixture was washed at 60° C. with 5 g of water to extract the total of DBSA-Na in the aqueous layer. In the aqueous extraction layer, 1 mol %/(the feed) of DMi was lost. After the extraction, xylene was similarly distilled off from the organic layer, and 9.83 g of DBP, 40 g of xylene and 1 mol % of DMi were added. Next, the solution was heated until reflux began, and azeotropic dehydration was carried out, while 3.98 g of a 49 wt % aqueous NaOH solution was added dropwise. Afterward, in accordance with the same procedure as in Example 4, reaction was carried out. The resulting wet DBSA-Na was dissolved in 54 g of water at 60° C., and the solution was then mixed with the aqueous extraction layer of the above recovered mixture of the filtrate and the wash liquid. The solution was washed with xylene, and then separated to obtain an aqueous layer. Xylene dissolved in the aqueous layer was distilled off under reduced pressure, and the solution was then added dropwise to 49 g of a 6% sulfuric acid solution over 2 hours to carry out acidifying-out, thereby obtaining DBSA. In consequence, purity was 99.8%, and yield was 98.1%/(addition DBP). Afterward, the similar operation was repeated 6 times, but problems did not occur with regard to reaction yield, recovery yield, product purity and DMi loss percent.
References:
US6392090,2002,B1 Location in patent:Page column 8,9
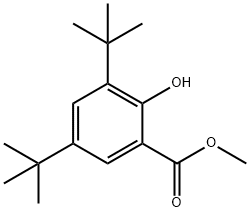
15018-03-8
22 suppliers
$118.00/10g

19715-19-6
197 suppliers
$8.00/5g
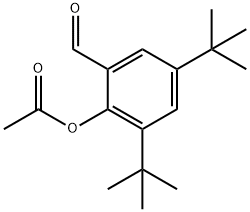
173030-55-2
0 suppliers
inquiry

19715-19-6
197 suppliers
$8.00/5g
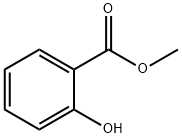
119-36-8
969 suppliers
$5.00/10g

19715-19-6
197 suppliers
$8.00/5g
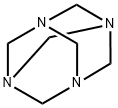
100-97-0
7 suppliers
$14.00/250G
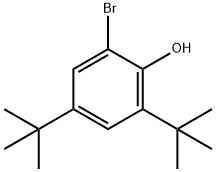
20834-61-1
65 suppliers
$45.00/25mg
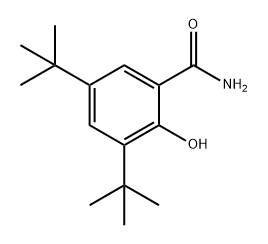
1044260-52-7
0 suppliers
inquiry

19715-19-6
197 suppliers
$8.00/5g
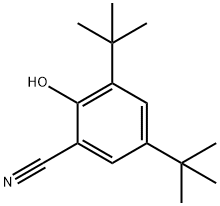
95091-86-4
1 suppliers
inquiry
![Phenol, 2-[[6,8-bis(1,1-dimethylethyl)-2H-1,3-benzoxazin-3(4H)-yl]methyl]-4,6-bis(1,1-dimethylethyl)-](/CAS/20210305/GIF/120695-69-4.gif)
120695-69-4
0 suppliers
inquiry