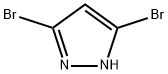
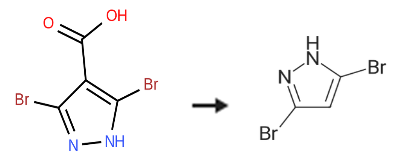
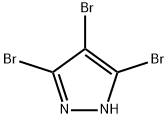
3,5-Dibromo-1H-pyrazole synthesis
- Product Name:3,5-Dibromo-1H-pyrazole
- CAS Number:67460-86-0
- Molecular formula:C3H2Br2N2
- Molecular Weight:225.87
17635-44-8
164 suppliers
$12.00/5g

67460-86-0
89 suppliers
inquiry
Yield:67460-86-0 86%
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane at -78; for 1 h;
Steps:
1.1 Step 1: 3,5-Dibromo-1 H-pyrazole
To a solution of 3,4,5-tribromo-1H-pyrazole (25 g, 81.96 mmol, 1.0 eq) in THF (300 ml), was added n-BuLi (2.5 M in hexanes, 65.6 ml, 164 mmol, 2.0 eq) over 30 min at -78° C. and the RM was stirred at this temperature for 30 min.
The RM was quenched by the dropwise addition of MeOH-THF (2:3; 125 ml) at -78° C., and stirred for an additional 1.5 h while gradually allowing it to warm to RT.
The solvent was removed under reduced pressure.
The residue was diluted with diethyl ether (600 ml), washed with aq. HCl (0.5 N, 60 ml) and brine (75 ml), dried (Na2SO4), filtered and concentrated under reduced pressure to afford the title product (16 g, 86%)
References:
GRÜNENTHAL GMBH;Schunk, Stefan;Reich, Melanie;Koenigs, René Michael US2017/101397, 2017, A1 Location in patent:Paragraph 0202

98026-81-4
5 suppliers
inquiry

67460-86-0
89 suppliers
inquiry