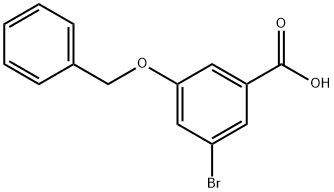
3-Benzyloxy-5-bromobenzoic acid synthesis
- Product Name:3-Benzyloxy-5-bromobenzoic acid
- CAS Number:1242336-70-4
- Molecular formula:C14H11BrO3
- Molecular Weight:307.14
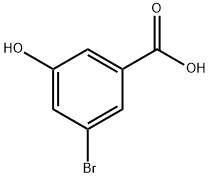
140472-69-1
173 suppliers
$19.00/250mg

100-39-0
424 suppliers
$10.00/10g

1242336-70-4
15 suppliers
$60.00/100mg
Yield:1242336-70-4 78%
Reaction Conditions:
Stage #1: 3-bromo-5-hydroxy-benzoic acid;benzyl bromidewith potassium carbonate in N,N-dimethyl-formamide at 20; for 24 h;
Stage #2: with potassium hydroxide in methanol; for 1 h;Reflux;
Stage #3: with hydrogenchloride in methanol;water; pH=2 - 3;
Steps:
1
The 5-hydroxy-3-bromo-benzoic acid (1 g, 4.6 mmol), K2C03 (1.33 g, 9.66 mmol), and BnBr (1.61 g, 9.43 mmol) were dissolved in dry DMF (10 mL) and stirred for 24 h. Water was added to the reaction mixture and extracted with EtOAc thrice (3 x 10 mL). The combined organic phase was evaporated and dissolved in 8N KOH in MeOH (50 mL) and refluxed for another 1 h. After completion of the reaction, the mixture was acidified with concentrated HCl to pH 2-3. The formed precipitate was filtered, dissolved in EtOAc and washed with water, brine and dried over Na2S04. Removal of the solvent gave pure product as a light yellow color solid, m.p. 148-150°C (1.1 g, 78% yield). NMR (400 MHz, d6- Acetone) δ: 1 1.8 - 10.8 (brs, 1H), 7.78, (s, 1H), 7.62, (s, 1H), 7.55 - 7.2 (m, 6H), 5.21 (s, 2H).
References:
WO2012/171114,2012,A1 Location in patent:Page/Page column 70

140472-69-1
173 suppliers
$19.00/250mg

1242336-70-4
15 suppliers
$60.00/100mg

100-39-0
424 suppliers
$10.00/10g

1242336-70-4
15 suppliers
$60.00/100mg