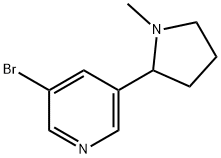
3-BroMo-5-(1-Methylpyrrolidin-2-yl)pyridine synthesis
- Product Name:3-BroMo-5-(1-Methylpyrrolidin-2-yl)pyridine
- CAS Number:71719-09-0
- Molecular formula:C10H13BrN2
- Molecular Weight:241.13
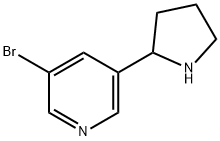
71719-06-7
53 suppliers
$125.00/50mg

71719-09-0
12 suppliers
inquiry
Yield:71719-09-0 95%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;formaldehyd;sodium cyanoborohydride;magnesium sulfate;acetic acid in acetonitrile;
Steps:
4 5-Bromo-3-(1-methyl-2-pyrrolidinyl)pyridine
EXAMPLE 4 5-Bromo-3-(1-methyl-2-pyrrolidinyl)pyridine To a solution of 5-bromo-3-(2-pyrrolidinyl) pyridine (18.14 g, 80.6 mmol) in acetonitrile (250 mL) at a temperature of 0° C. was added an aqueous solution of formaldehyde (60.4 mL, 37% by weight, 806 mmol) and the mixture was stirred for 20 min. Solid sodium cyanoborohydride (7.60 g, 120 mmol) was added in several portions over 30 min, and the reaction mixture was stirred at 0° C. for an additional 90 min, then 3.0 mL of acetic acid was added and the reaction was allowed to warm to room temperature and stirred for 15 h. The reaction mixture was diluted with 75 mL of 1M aqueous hydrochloric acid and the organic solvents were removed by rotary evaporation. The residue was adjusted to pH 2.5 by the addition of 1N HCl and extracted three times with 75 mL portions of methylene chloride. The aqueous phase was basified to pH 12 by the addition of solid sodium hydroxide and extracted three times with 75 mL portions of methylene chloride. The organic phases from the basic extraction were combined and treated with magnesium sulfate and activated charcoal, then filtered through Celite. The solvent was removed by rotary evaporation, and the residual solvent was removed under high vacuum to provide 5-bromo-3(1-methyl-2-pyrrolidinyl)pyridine (18.19 g, 95%) as a pale yellow oil LRMS (EI)m/e 242 (C10 H13 N281 Br) 241 (C10 H13 N279 Br--+ H), 240 (C10H13 N279 Br), 239 (C10 H13 N281 Br--+ H); 1 H NMR (DMSO-d6, 300 MHz) δ 8.55 (d, J=2.1 Hz, 1 H), 8.44 (d, J=1.9 Hz, 1 H), 7.88 (t, J=1.9 Hz, 1 H), 3.24 (bd-t, J=8.1 Hz, 1 H), 3.10 (t, J=8.0 Hz, 1 H), 2.36 (m, 1 H), 2.18 (s, 3 H), 1.95 (m, 1 H), 1.85 (m, 1 H), 1.70 (m, 1 H).
References:
US5594011,1997,A

50-00-0
842 suppliers
$10.00/25g

71719-06-7
53 suppliers
$125.00/50mg

71719-09-0
12 suppliers
inquiry

1253523-01-1
0 suppliers
inquiry

71719-09-0
12 suppliers
inquiry
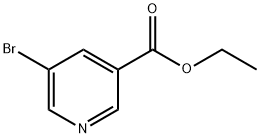
20986-40-7
271 suppliers
$6.00/10g

71719-09-0
12 suppliers
inquiry
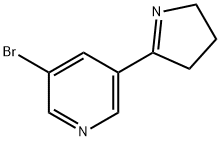
64319-85-3
13 suppliers
inquiry

71719-09-0
12 suppliers
inquiry