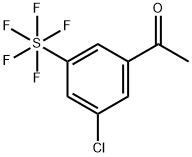
3'-Chloro-5'-(pentafluorosulfur)acetophenone synthesis
- Product Name:3'-Chloro-5'-(pentafluorosulfur)acetophenone
- CAS Number:1180486-96-7
- Molecular formula:C8H6ClF5OS
- Molecular Weight:280.65
1178585-27-7
0 suppliers
inquiry

1180486-96-7
11 suppliers
$208.00/1g
Yield:-
Reaction Conditions:
Stage #1: 1-[3-amino-5-(pentafluorosulfanyl)phenyl]ethanonewith hydrogenchloride;sodium nitrite in water at 5;Inert atmosphere;
Stage #2: with hydrogenchloride;copper(l) chloride in water at 0 - 25;Inert atmosphere;
Steps:
27.27a
1-[3-Amino-5-(pentafluorosulfanyl)phenyl]ethanone (150 mg, ex. 22a) was dissolved in semiconcentrated hydrochloric acid (6 ml). While stirring and cooling, sodium nitrite solution (40 mg dissolved in water) was added dropwise, in the course of which the temperature was not to exceed 5° C. On completion of formation of the diazonium salt, copper(I) chloride solution (68 mg dissolved in 2 ml of concentrated hydrochloric acid) was cooled to 0° C. while stirring and the above diazonium salt solution was slowly added dropwise. Then the cooling bath was removed, and the mixture was stirred at RT for 3 h and left to stand overnight. Then the reaction mixture was diluted with water and extracted repeatedly with EA. The combined EA phases were washed with saturated sodium hydrogencarbonate solution, dried over magnesium sulfate, filtered and concentrated. The residue was purified by means of preparative HPLC. The product-containing fractions were combined, freed of the acetonitrile, admixed with saturated sodium hydrogencarbonate solution and extracted three times with ethyl acetate. The combined extracts were dried over magnesium sulfate, filtered and concentrated. 104 mg of the desired compound were obtained.1H NMR (400 MHz, DMSO-d6) [ppm]: 8.38 (1H); 8.32 (1H); 8.22 (1H); 2.67 (3H)
References:
US2011/34461,2011,A1 Location in patent:Page/Page column 34

833-96-5
32 suppliers
$33.00/500mg

1180486-96-7
11 suppliers
$208.00/1g
852469-45-5
0 suppliers
inquiry

1180486-96-7
11 suppliers
$208.00/1g