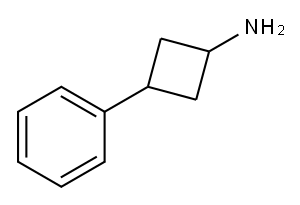
3-phenylcyclobutan-1-amine synthesis
- Product Name:3-phenylcyclobutan-1-amine
- CAS Number:90874-41-2
- Molecular formula:C10H13N
- Molecular Weight:147.22
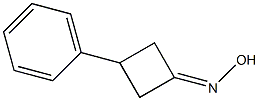
99059-91-3
0 suppliers
inquiry

90874-41-2
35 suppliers
$250.00/250mg
Yield:-
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran; for 4 h;Reflux;
Steps:
Synthesis of cis/trans-3-Phenylcyclobutylamine (28)
To a solution of 330 mg 27 (2 mmol) in 10 cm3 dry THF,4.41 cm3 1 M solution of LiAlH4 in THF (4.41 mmol) was added dropwise over a period of 3 min. The reaction was heated to reflux for 4 h. After cooling, 2 cm3 0.5 M solution of NaOH was added and the mixture was stirred for 30 min. The suspension was filtered, and the solid was washed with 3*5 cm3 THF. The filtrate was concentrated under reduced pressure and the residue was dissolved in 25 cm3 1 M HCl. The resulting solution was washed with 3*15 cm3 diethyl ether; the water layer was neutralized with 1 M NaHCO3 and extracted with 3*20 cm3 CH2Cl2. The organic layers were combined, dried over Na2SO4 and concentrated in vacuo. Colourless oil; yield: 144 mg (49%). 1H NMR (400 MHz, CDCl3): trans isomer: δ = 1.68 (bs, 2 H), 1.78 (m, 2H), 2.73 (m, 2H), 3.05 (m,1H), 3.39 (m, 1H), 7.23 (m, 5H) ppm; cis isomer: δ = 1.68(bs, 2 H), 2.17 (m, 2H), 2.46 (m, 2H), 3.59 (m, 1H), 3.67(m, 1H), 7.23 (m, 5H) ppm. The 1H NMR spectrum was found to be similar to the one described for the corresponding hydrochloride of 28 in Ref. [54].
References:
Horáková, Eva;Drabina, Pavel;Br??ková, Lenka;?těpánková, ?árka;Vor?áková, Katarína;Sedlák, Milo? [Monatshefte fur Chemie,2017,vol. 148,# 12,p. 2143 - 2153]
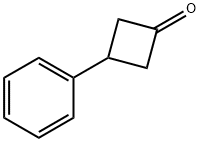
52784-31-3
92 suppliers
$16.00/250mg

90874-41-2
35 suppliers
$250.00/250mg
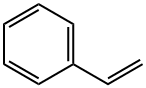
100-42-5
617 suppliers
$20.00/25mL

90874-41-2
35 suppliers
$250.00/250mg