
3-(PHENYLTHIO)PROPAN-1-AMINE HYDROCHLORIDE synthesis
- Product Name:3-(PHENYLTHIO)PROPAN-1-AMINE HYDROCHLORIDE
- CAS Number:78540-47-3
- Molecular formula:C9H14ClNS
- Molecular Weight:203.73
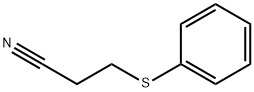
3055-87-6
8 suppliers
inquiry

78540-47-3
11 suppliers
inquiry
Yield:78540-47-3 37%
Reaction Conditions:
Stage #1: 3-(phenylsulfanyl)propanenitrilewith sodium tetrahydridoborate;CoCl2·6H2O in methanol at 20; for 1 h;
Stage #2: with hydrogenchloride in ethanol;ethyl acetate;
Steps:
3-(phenylsulfanyl)propan-1-amine hydrochloride (SZV-2016)
To a solution of 3-(phenylsulfanyl)propanenitrile (prepared according to [6] (5.71 g, 35.00 mmol) in methanol (210mL) CoCl2×6H2O (16.66 g, 70.00 mmol, 2.0 equiv) was added. Upon cooling NaBH4 (13.30 g, 350.00 mmol, 10.0 equiv)was added in small portions. After the addition of the reagent the mixture was stirred at rt for 1 h. The reaction wasquenched with 3 M HCl (110 mL) and the methanol was distilled off. The residue was extracted with diethyl ether. ThepH of the aqueous phase was adjusted to >10 with 3% NaOH and it was extracted with DCM. The combined DCMphases were evaporated to dryness and purified with column chromatography (CHCl3:MeOH:H2O 60:12:1) to yieldthe amine product (2.19 g, 37%). A portion of the amine product (0.73 g, 4.36 mmol) was dissolved in EtOAc andtreated with EtOAc/HCl to yield the hydrochloride salt. White crystals (0.78 g, quant.), mp 163.0-167.0°C. Anal.(C9H14ClNS) calcd. C 53.06; H 6.93; N 6.88. Found: C 51.98; H 6.49; N 6.74.
References:
Carpéné, Christian;Viana, Pénélope;Iffiú-Soltesz, Zsuzsa;Tapolcsányi, Pál;F?ldi, Anna ágota;Mátyus, Péter;Dunkel, Petra [Molecules,2022,vol. 27,# 19,art. no. 6224] Location in patent:supporting information
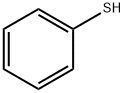
108-98-5
4 suppliers
$21.10/10g

78540-47-3
11 suppliers
inquiry
