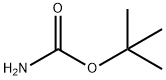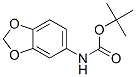
Carbamic acid, 1,3-benzodioxol-5-yl-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, 1,3-benzodioxol-5-yl-, 1,1-dimethylethyl ester (9CI)
- CAS Number:333749-47-6
- Molecular formula:C12H15NO4
- Molecular Weight:237.2518

24424-99-5
816 suppliers
$13.50/25G
14268-66-7
268 suppliers
$20.00/5G

333749-47-6
8 suppliers
inquiry
Yield:333749-47-6 75%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 20; for 16 h;
Steps:
[int-1.32] tert-Butyl N-(1 ,3-benzodioxol-5-yl)carbamate:
To a solution of 1,3-benzodioxol-5-amine (10.0 g, 73.0 mmol) in DCM (100 mL) were added tert-butoxycarbonyl tert-butyl carbonate (19.0 g, 87.6 mmol) and DIPEA (19 mL, 109.5 mmol), and the mixture stirred at room temperature for 16 h. Thesolution was washed with sat. aq. NH4C1 (100 mL) and brine (100 mL), dried over anhydrous Na2504, filtered and the solvent evaporated under reduced pressure. The resultant residue was purified by silica gel flash-column chromatography, with cyclohexane/EtOAc (9:1) as the eluent.The product was further purified by trituration, with cyclohexane as the solvent, to yield the title compound as a white solid (13.0 g, 75%) : ‘H NNR (400 MHz, DMSO-d6) 6 9.16 (s, 1H), 7.10 (d, J = 2.1 Hz, 1H), 6.85 (dd, J = 8.4, 2.1 Hz, 1H), 6.78 (d, J = 8.4 Hz, 1H), 5.94 (s, 2H), 1.46 (s, 9H); UPLC-MS: tR = 2.41 mm (generic method); MS (ESI) m/z calcd for C,2H,4N04 (M-H)-: 236.1, found: 236.2.
References:
WO2018/167695,2018,A1 Location in patent:Page/Page column 90; 91