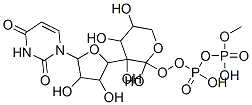
[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[hydroxy-(3,4,5-trihydroxyoxan-2-yl)oxy-phosphoryl]oxy-phosphinic acid synthesis
- Product Name:[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[hydroxy-(3,4,5-trihydroxyoxan-2-yl)oxy-phosphoryl]oxy-phosphinic acid
- CAS Number:3616-06-6
- Molecular formula:C14H22N2O16P2
- Molecular Weight:536.28
Yield:-
Reaction Conditions:
with UDP-api/UDP-xylose synthase from Arabidopsis thaliana;nicotinamide adenine dinucleotide in aq. phosphate buffer at 25; pH=8; for 8 h;Enzymatic reaction;
Steps:
Enzymatic synthesis of UDP-Api
General procedure: The recombinant AtAXS1 protein was prepared as described previously[17]. The enzymatic synthesis of UDP-Api was carried out in areaction mixture comprising 100mM triethylamine phosphate buffer,pH 8.0, 50mM UDP-GlcUA, 0.5mM NAD+ and 10 μg/μL of the purifiedprotein. The reaction mixtures were incubated at 25 °C for 4 h and thereaction was terminated by adding phenol/chloroform/isoamyl alcohol (25:24:1, v/v/v), followed by vigorous shaking. The water phase containingthe reaction products was obtained by centrifugation(22,000×g at 4 °C for 5 min) and subjected to analysis.The reaction products were separated and monitored by a reversedphase (RP)eHPLC equipped with a UV detector. RP-HPLC was performedusing an Inertsil ODS-3 column (4.6×250 mm; GL Sciences,Tokyo, Japan) with an L-6200 intelligent pump and L-4000 UV detector(Hitachi High-Technologies, Tokyo, Japan) at a flow rate of 1.2 mL/min. The mobile phase was composed of 100mMN,N-dimethylcyclohexylaminephosphate buffer, pH 6.5. The eluted fractions were monitoredby measuring the absorbance at 262 nm.
References:
Fujimori, Tae;Matsuda, Ryoko;Suzuki, Mami;Takenaka, Yuto;Kajiura, Hiroyuki;Takeda, Yoichi;Ishimizu, Takeshi [Carbohydrate Research,2019,vol. 477,p. 20 - 25]
![(2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic](/CAS/20210111/GIF/2616-64-0.gif)
2616-64-0
50 suppliers
inquiry
![[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[hydroxy-(3,4,5-trihydroxyoxan-2-yl)oxy-phosphoryl]oxy-phosphinic acid](/CAS/GIF/3616-06-6.gif)
3616-06-6
23 suppliers
inquiry
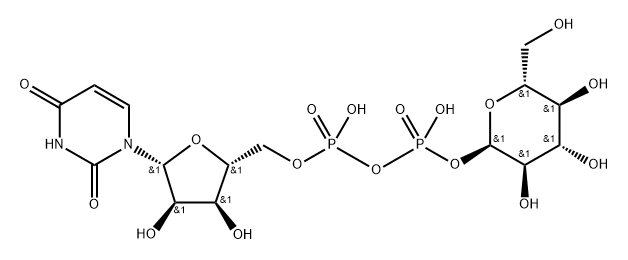
133-89-1
34 suppliers
inquiry
![[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[hydroxy-(3,4,5-trihydroxyoxan-2-yl)oxy-phosphoryl]oxy-phosphinic acid](/CAS/GIF/3616-06-6.gif)
3616-06-6
23 suppliers
inquiry
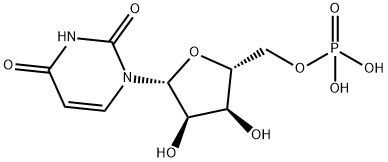
58-97-9
300 suppliers
$9.00/100mg
![[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[hydroxy-(3,4,5-trihydroxyoxan-2-yl)oxy-phosphoryl]oxy-phosphinic acid](/CAS/GIF/3616-06-6.gif)
3616-06-6
23 suppliers
inquiry
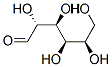
2280-44-6
7 suppliers
inquiry
![[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[hydroxy-(3,4,5-trihydroxyoxan-2-yl)oxy-phosphoryl]oxy-phosphinic acid](/CAS/GIF/3616-06-6.gif)
3616-06-6
23 suppliers
inquiry