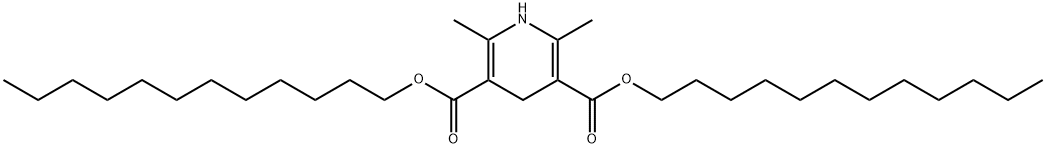
didodecyl 1,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylate synthesis
- Product Name:didodecyl 1,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylate
- CAS Number:36265-41-5
- Molecular formula:C33H59NO4
- Molecular Weight:533.8259
Yield:36265-41-5 92.1%
Reaction Conditions:
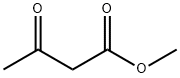
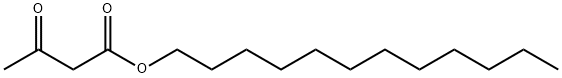
Stage #1: 1-dodecyl alcohol;methyl 3-oxobutanoate at 160;
Stage #2: with formalin;ammonium acetate in methanol;lithium hydroxide monohydrate;Reflux;Reagent/catalyst;
Steps:
1-10 Example 1
Add 400kg of lauryl alcohol and 1000kg of methyl acetoacetate into the reaction kettle, stir and heat up to 160±5°C, and put it in the reactor.The reaction is kept at this temperature until the reaction is complete, and the methanol generated by the reaction is recovered during the reaction. After the reaction, the reaction system was first cooled to 90±5°C, and then the excess methyl acetoacetate was evaporated under reduced pressure,Then, the temperature was lowered to below 60° C. to obtain 558 kg of orange-brown transparent liquid, which was ready for use. The steamed methyl acetoacetate is reused to the next batch. 2. add 120kg of water, 100kg of methanol (wherein 50kg is derived from step 1. recovered formazan in the reactor successively)alcohol), 165.6kg urotropine,45.6kg of ammonium acetate and 558kg of orange-brown transparent liquid obtained in step ,then heat upIncubate the reaction at reflux to complete. After the reaction is completed, the temperature is lowered to 65-70°C, 500kg of water is added first, then 1200kg of toluene is added, and the mixture is left to stand after stirring.The organic phase was cooled to 30°C, suction filtered, centrifuged, dried,516kg of light yellow DHP was obtained,The purity is 98.5% (HPLC),The overall yield was 90.0%.
References:
CN108299286,2018,A Location in patent:Paragraph 0015-0028

112-53-8
415 suppliers
$10.00/5g

36265-41-5
41 suppliers
inquiry