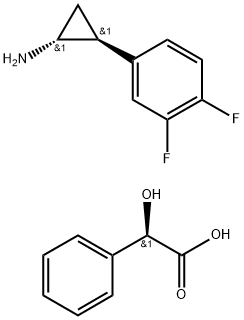
(1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2R)-Hydroxy(phenyl)ethanoate synthesis
- Product Name:(1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2R)-Hydroxy(phenyl)ethanoate
- CAS Number:376608-71-8
- Molecular formula:C17H17F2NO3
- Molecular Weight:321.32
2. The resulting residue was dissolved in dichloromethane (1075 ml) and then cooled to 10 to 15° C. 25% Aqueous ammonia solution (1290 ml) was added to the cooled solution while maintaining the temperature at below 30° C. The resulting reaction mass was stirred for 15 minutes, followed the by layer separation. The resulting aqueous layer was extracted with dichloromethane (2×537.5 ml) and then combined with the main dichloromethane layer. The combined dichloromethane layer containing the product was extracted thrice with aqueous hydrochloric acid (645 ml of conc. hydrochloric acid mixed with 1935 ml water, 3×865 ml). The aqueous acidic layer containing the product was combined and washed with dichloromethane (645 ml). Dichloromethane (1075 ml) was added to the acidic aqueous layer, followed by the addition of 25% aqueous ammonia solution (1505 ml) while maintaining the temperature at below 30° C. The resulting reaction mass was extracted with dichloromethane (2×645 ml) and then combined with the main dichloromethane layer. The combined dichloromethane layer containing the product was washed with water (645 ml) and evaporated to dryness under reduced pressure.
3. The resulting residue was dissolved in methanol (430 ml), followed slow addition of (R)-(-)-mandelic acid solution (107.5 g in 645 ml methanol) over a period of 40 to 60 minutes while maintaining temperature at 20 to 25° C. The resulting slurry was stirred further for 12 hours at 20 to 25° C., followed by further cooling to 0 to 5° C. The cooled solution was stirred for 2 hours and the solid was isolated by filtration. The resulting solid was washed with chilled methanol (215 ml). The solid was dried under reduced pressure at 40 to 45° C. to obtain 127 g of pure (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2R)-Hydroxy(phenyl)ethanoate as a white solid.
220352-36-3
107 suppliers
inquiry
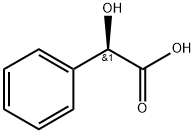
611-71-2
612 suppliers
$13.00/5g

376608-71-8
402 suppliers
$5.00/250mg
Yield:376608-71-8 82%
Reaction Conditions:
Stage #1: trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxylic acidwith pyridine;thionyl chloride in toluene at 70; for 4 h;
Stage #2: with sodium azide;tetrabutylammomium bromide;sodium carbonate in water;toluene at 0; for 2 h;
Stage #3: (R)-Mandelic Acid in ethyl acetate at 17 - 25; for 3 h;
Steps:
2 Example 2Compound of formula II is prepared by rearrangement reaction and salt-forming one-pot method
Dissolve 3.45 g of the compound of formula II in 15 mL of toluene, add a drop of pyridine,Add 2.3g of thionyl chloride, warm to 70 ° C, and keep for three hours,Add another 0.5g of Thionyl chloride and keep it warm for another 1h. The reaction solution was evaporated to dryness,Use 15g * 3 toluene with sulfoxide chloride residue. Dissolve the residue in 10mL toluene,Cool to 0 , add 1.24g sodium azide, 0.056g tetrabutylammonium bromide,A 6.2g aqueous solution of 0.922g sodium carbonate was kept for 2h. Add 3.8g water to dilute,Separate the layers, wash the organic layer with 3.8g, 3.8mL saturated sodium chloride,3 for use. Heat 6g of toluene to 100 ° C, add the solution to be used dropwise,Incubate for 1 hour after dropping. Cool down to 20 ° C.Add the above solution dropwise to 18.2g of 3M HCl solution, control the temperature at 80 ,Reaction 65min. 34g of water was added, the reaction liquid was lowered to 25 ° C, and the layers were separated.The water layer was adjusted to pH 12 with 3.8g 45% sodium hydroxide solution,Extract with 31g of ethyl acetate, separate the layers, wash the ethyl acetate layer with 13.7mL * 2 water,stand-by. Dissolve 2.26g R-mandelic acid in 45.3mL ethyl acetate,Add dropwise to the above-mentioned to-be-used solution, and control the temperature to 17 ° C.After the drop, stir at 25 ° C for 3h, filter with suction, and wash the filter cake with 13.8g of ethyl acetate.Dry at 40 ° C to obtain 4.54g of compound of formula III,The yield was 82%, and the ee value was> 99.9%.
References:
CN111072467,2020,A Location in patent:Paragraph 0014
![Benzene, 1,2-difluoro-4-[(1S,2R)-2-nitrocyclopropyl]-](/CAS/20200515/GIF/1345413-26-4.gif)
1345413-26-4
1 suppliers
inquiry

611-71-2
612 suppliers
$13.00/5g

376608-71-8
402 suppliers
$5.00/250mg
367-11-3
306 suppliers
$10.00/1g

376608-71-8
402 suppliers
$5.00/250mg
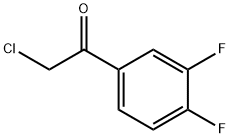
51336-95-9
237 suppliers
$15.00/1g

376608-71-8
402 suppliers
$5.00/250mg

1215969-79-1
3 suppliers
inquiry

376608-71-8
402 suppliers
$5.00/250mg