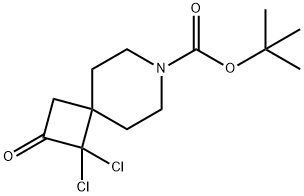
tert-butyl 1,1-dichloro-2-oxo-7-azaspiro[3.5]nonane-7-carboxylate synthesis
- Product Name:tert-butyl 1,1-dichloro-2-oxo-7-azaspiro[3.5]nonane-7-carboxylate
- CAS Number:396653-31-9
- Molecular formula:C13H19Cl2NO3
- Molecular Weight:308.2
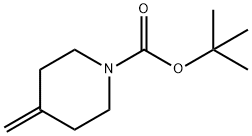
159635-49-1
248 suppliers
$5.00/250mg
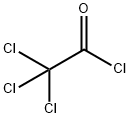
76-02-8
249 suppliers
$38.29/25G
![tert-butyl 1,1-dichloro-2-oxo-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/20180601/GIF/396653-31-9.gif)
396653-31-9
13 suppliers
inquiry
Yield:396653-31-9 91%
Reaction Conditions:
with zinc-copper couple in 1,2-dimethoxyethane at 34 - 44;Inert atmosphere;
Steps:
Synthesis of tert-butyl 1,1-dichloro-2-oxo-7-azaspiro[3.5]nonane-7-carboxylate; Dry DME (8.0 L) and tert-butyl 4-methylenepiperidine-1-carboxylate (800 g, 4.06 mol) were charged to a reactor. Zinc-copper couple (800 g; CAS No.53801-63-1, Alfa-Aesar) was charged to the reactor, and the mixture was warmed to 34° C. Trichloroacetyl chloride (1448 g, 8.0 mol, 888 mL) was added dropwise under a nitrogen atmosphere to the stirred suspension in the following manner: 80 mL of trichloroacetyl chloride was added. After 10 min, an exotherm elevated the reaction temperature to 39° C. Dropwise addition of the remaining trichloroacetyl chloride was resumed immediately at a rate to maintain a temperature between 40-44° C. using a 25° C. jacket. After the addition was complete, the reaction was stirred at 40° C. for 15 min. Cyclohexane (10 L) was added to the mixture. The mixture was filtered through a pad of celite, washing with cyclohexane (2 L). The filtrate was concentrated to approximately 3 L and then was diluted with MTBE (3 L) and cyclohexane (2 L) and filtered through a pad of magnesol (1 kg), washing with 1:1 cyclohexane/MTBE (3 L). The filtrate was washed with saturated potassium bicarbonate (3 L) and brine (2 L). The organic layer was filtered through a pad of silica gel (300 g) with a pad of magnesol (200 g) on top. The filtrated was concentrated to yield the title compound as an orange solid (1123 g, 91%). 1H NMR (400 MHz, CDCl3) δ ppm 4.05-4.13 (m, 2H), 3.08 (s, 2H), 2.80-2.88 (m, 2H), 1.88-1.97 (m, 2H), 1.71-1.78 (m, 2H), 1.46 (s, 9H). m/z 252, 254 (MH+ minus t-Bu).
References:
US2010/113465,2010,A1 Location in patent:Page/Page column 15

144-55-8
1306 suppliers
$10.00/25g

159635-49-1
248 suppliers
$5.00/250mg

76-02-8
249 suppliers
$38.29/25G
![tert-butyl 1,1-dichloro-2-oxo-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/20180601/GIF/396653-31-9.gif)
396653-31-9
13 suppliers
inquiry