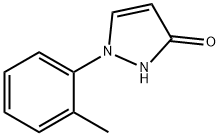
3H-Pyrazol-3-one, 1,2-dihydro-1-(2-methylphenyl)- synthesis
- Product Name:3H-Pyrazol-3-one, 1,2-dihydro-1-(2-methylphenyl)-
- CAS Number:1242307-76-1
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
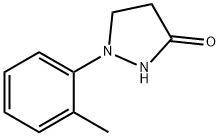
6112-49-8
0 suppliers
inquiry

1242307-76-1
1 suppliers
inquiry
Yield:1242307-76-1 80%
Reaction Conditions:
with iodine in dimethyl sulfoxide at 100; for 18 h;
Steps:
7 Synthesis of N-arylpyrazole compound 2g (see Fig. 8 for the synthetic route):
In a 25 mL reaction tube, add a magnetic stir bar and iodine (5.0 mol%, 2.5 mg),1 g (0.20 mmol, 35.2 mg) of N-aryl-3-pyrazolidone compound was weighed and added,Then add dimethyl sulfoxide solvent (2.0mL) to dissolve, cover the reaction tube cap,Transfer it to a 100°C oil bath under the reaction conditions of air,The reaction was carried out for 18 hours (monitored by thin layer chromatography). After the reaction of 1 g of the raw material is completed,The reaction tube was cooled to room temperature, and then saturated sodium thiosulfate solution (5.0 mL) was added,Extract 3 times with ethyl acetate (5.0 mL), take the organic layer solution, dry it with anhydrous sodium sulfate,The solvent was evaporated to dryness, then the crude product was separated by column chromatography (eluent, petroleum ether:ethyl acetate=5:1, volume ratio),The N-arylpyrazole compound 2g (28.0 mg, yield 80%) was obtained.
References:
CN114456117,2022,A Location in patent:Paragraph 0064-0066
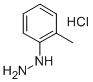
635-26-7
194 suppliers
$5.00/1g

1242307-76-1
1 suppliers
inquiry
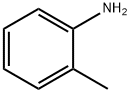
95-53-4
431 suppliers
$10.00/1g

1242307-76-1
1 suppliers
inquiry