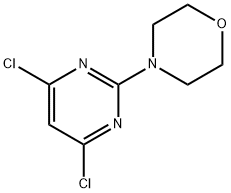
4-(4,6-Dichloropyrimidin-2-yl)morpholine synthesis
- Product Name:4-(4,6-Dichloropyrimidin-2-yl)morpholine
- CAS Number:10397-13-4
- Molecular formula:C8H9Cl2N3O
- Molecular Weight:234.08

110-91-8
673 suppliers
$9.00/1g
3764-01-0
398 suppliers
$6.00/5g

10397-13-4
167 suppliers
$11.00/250mg
52127-83-0
68 suppliers
$95.00/1 g
Yield: 79% , 20%
Reaction Conditions:
in acetone at 0 - 20; for 0.5 h;
Steps:
3.1.4. 4-(2,6-Dichloropyrimidin-4-yl)morpholine, (10) and 4-(4,6-dichloropyrimidin-2-yl)morpholine, (11)
2,4,6-Trichloropyrimidine (5) (3.158 g, 17.22 mmol) and acetone (60 mL) were combined and cooledto 0 °C. Morpholine (7) (1.05 eq., 1.576 g, 18.09 mmol) was added and the solution stirred at 0 °C for15 min then warmed to room temperature for another 15 min. The reaction was monitored by TLCanalysis (20% ethyl acetate in hexanes). The reaction was then concentrated via rotary evaporationand further dried under high vacuum. Product was purified using silica column chromatographywith 20% ethyl acetate in hexanes as the eluent. 10 (major regioisomer) (3.183 g, 13.6 mmol, 79%).Elem. anal. for C8H9N3OCl2: C, 41.05 (found 42.27); H, 3.88 (found 4.06). 1H-NMR (300 MHz, CDCl3) δ 6.40 (s, 1H), 3.82-3.70 (m, 4H), 3.65 (m, 4H). HRMS [M]+ calcd. for C8H9N3OCl2, 234.0201; found234.0196. 11 (minor regioisomer) (0.806 g, 3.444 mmol, 20%). 1H-NMR (300 MHz, chloroform-d) δ 6.56 (s, 1H), 3.85-3.60 (m, 8H). 13C-NMR (101 MHz, chloroform-d) δ 161.75, 160.55, 108.31, 66.59, 44.39.HRMS [M]+ calcd. for C8H9N3OCl2, 234.0201; found 234.0196.
References:
Wright, Emily W.;Nelson, Ronald A.;Karpova, Yelena;Kulik, George;Welker, Mark E. [Molecules,2018,vol. 23,# 7,p. 1 - 13]

110-91-8
673 suppliers
$9.00/1g

3764-01-0
398 suppliers
$6.00/5g

10397-13-4
167 suppliers
$11.00/250mg

5414-19-7
189 suppliers
$6.00/5g
56-05-3
436 suppliers
$10.00/1g

10397-13-4
167 suppliers
$11.00/250mg
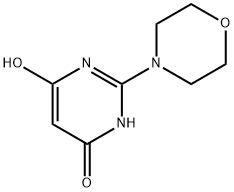
24193-00-8
48 suppliers
$121.00/1g

10397-13-4
167 suppliers
$11.00/250mg
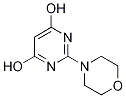
50596-83-3
2 suppliers
inquiry

10397-13-4
167 suppliers
$11.00/250mg