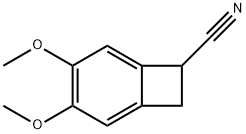
4,5-Dimethoxy-1-cyanobenzocyclobutane synthesis
- Product Name:4,5-Dimethoxy-1-cyanobenzocyclobutane
- CAS Number:35202-54-1
- Molecular formula:C11H11NO2
- Molecular Weight:189.21
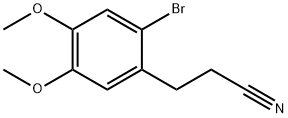
35249-62-8
57 suppliers
inquiry

35202-54-1
256 suppliers
$9.00/250mg
Yield:35202-54-1 80%
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane at -10;Inert atmosphere;
Steps:
1
Example 1.Preparation of 3,4-dimethoxy-bicyclo[4.2.0]octa-l,3,5-triene-7-carbonitrile (III)In a 250 ml flask in argon atmosphere 50 ml of anhydrous tetrahydrofuran was added. The mixture was cooled to -10 °C and 33 ml of 2.5 M butyllithium hexane solution was added 25 dropwise while the temperature was kept below-10 °C. Then the reaction was stirred for 10 minutes at -10°C. 13.0 ml of diisopropylamine (or 10.0 ml diethylamine) was added dropwise while the temperature was kept at -10°C.Then the reaction was stirred for 10 minutes. 10.0 g of 3-(2-bromo-4,5-dimethoxy- phenyl)-propionitrile (II) dissolved in 30 ml of anhydrous tetrahydrofuran was added30 dropwise to the solution at -10 °C. The reaction was stirred at -10 °C.After the reaction had been completed it was allowed to warm up to 0 °C and 100 ml of 1 M hydrochloric acid was added dropwise while the temperature was kept below 20 °C. After 5 minutes stirring the phases were separated. The aqueous phase was washed twice with 50 ml of toluol. The combined organic phases were washed with 1x50 ml of 1 M hydrochloric acid and 1x50 ml of saturated sodium chloride solution.The organic phase was evaporated in vacuum to 20 g. 20 ml of ethanol was added to the residue and it was evaporated in vacuum again to 20 g. 30 ml of ethanol was added to the residue and it was evaporated again to 20 g.The residue was stirred on ice-water. The product was crystallized, it was stirred for 30 minutes at 0-5 °C. Then it was filtered and washed with ethanol. It was dried in vacuum at 40 °C.5.55 g of the title compound was obtained in 80% yield.
References:
WO2011/138625,2011,A1 Location in patent:Page/Page column 31-32
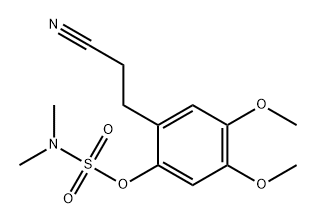
1232191-48-8
0 suppliers
inquiry

35202-54-1
256 suppliers
$9.00/250mg
![3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-one](/CAS/20180601/GIF/55171-76-1.gif)
55171-76-1
4 suppliers
inquiry
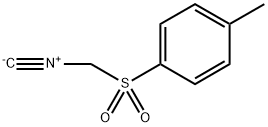
36635-61-7
490 suppliers
$5.00/5g

35202-54-1
256 suppliers
$9.00/250mg
![Bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile, 3,4-dimethoxy-, (7R)-](/CAS/20210305/GIF/1214788-46-1.gif)
1214788-46-1
0 suppliers
inquiry

35202-54-1
256 suppliers
$9.00/250mg
7721-62-2
54 suppliers
$45.00/25mg

35202-54-1
256 suppliers
$9.00/250mg