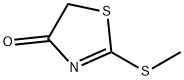
4(5H)-Thiazolone,2-(methylthio)-(9CI) synthesis
- Product Name:4(5H)-Thiazolone,2-(methylthio)-(9CI)
- CAS Number:20949-66-0
- Molecular formula:C4H5NOS2
- Molecular Weight:147.22
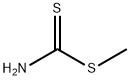
16696-83-6
9 suppliers
inquiry

79-04-9
353 suppliers
$12.00/5g

20949-66-0
21 suppliers
$221.00/500mg
Yield:20949-66-0 90%
Reaction Conditions:
with sodium hydrogencarbonate in acetonitrile at 81; for 12 h;
Steps:
4.2. General procedure for the preparation of 2-(alkylthio)thiazol-4(5H)-ones
General procedure: 4.2. General procedure for the preparation of 2-(alkylthio)thiazol-4(5H)-ones Dithiocarbamate (1mmol) and NaHCO3 (0.168 g, 2mmol) were mixed in acetonitrile(5 mL). Then, chloroacetyl chloride (0.08 mL, 1mmol) was added and the mixture wasrefluxed for 12 h at 81°C. Then, water (10 mL) was added and the product was extractedby ethyl acetate (3×10 mL). The organic layers were combined and dried with anhydrousMgSO4. Finally, evaporation of the solvent afforded the corresponding crude2-(alkylthio)thiazol-4(5H)-ones as a yellow oil. The purification was accomplished bycolumn chromatography (silicagel, n-hexane /ethyl acetate; 4:1). 2-(Methylthio)thiazol-4(5H)-one (3a): IR (KBr) 1724, 1695, 1438, 1385, 1188, 1023,955 cm-1; 1H NMR (500MHz, CDCl3) δ 2.70 (3H, s), 3.97 (2H, s) ppm; 13C NMR(125MHz, CDCl3) δ 16.8, 40.2, 187.9, 203.2 ppm; MS (EI): m/z 147 (M+), 123, 91(100),77, 69, 55, 45.
References:
Ziyaei Halimehjani, Azim;Alaei, M. Ali;Soleymani Movahed, Farzaneh;Jomeh, Negin;Saidi, Mohammad R. [Journal of Sulfur Chemistry,2016,vol. 37,# 5,p. 529 - 536]
141-84-4
255 suppliers
$8.00/5g

74-88-4
348 suppliers
$15.00/10g

20949-66-0
21 suppliers
$221.00/500mg

141-84-4
255 suppliers
$8.00/5g

74-88-4
348 suppliers
$15.00/10g
4807-55-0
49 suppliers
$76.29/5g

20949-66-0
21 suppliers
$221.00/500mg

6866-44-0
0 suppliers
inquiry

77-78-1
298 suppliers
$22.00/25g

20949-66-0
21 suppliers
$221.00/500mg

34705-87-8
1 suppliers
inquiry

74-88-4
348 suppliers
$15.00/10g

4807-55-0
49 suppliers
$76.29/5g

20949-66-0
21 suppliers
$221.00/500mg