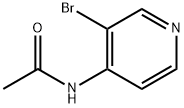
4-(Acetylamino)-3-bromopyridine synthesis
- Product Name:4-(Acetylamino)-3-bromopyridine
- CAS Number:13535-03-0
- Molecular formula:C7H7BrN2O
- Molecular Weight:215.05
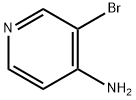
13534-98-0
259 suppliers
$6.00/250mg

75-36-5
559 suppliers
$17.92/100G

13535-03-0
4 suppliers
inquiry
Yield:13535-03-0 89%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 5 - 20; for 16 h;Inert atmosphere;
Steps:
5.1.25. N-(3-Bromopyridin-4-yl)acetamide (45)
3-Bromopyridin-4-amine (5.10 g, 29.48 mmol) and DIPEA (5.66 mL, 32.43 mmol) were dissolved in 6 mL CH2Cl2. Acetyl chloride (2.31 mL, 32.43 mmol) in 10 mL CH2Cl2 was added dropwise at 5 °C. The solution was stirred overnight, washed with H2O and concentrated in vacuo to give 45 (5.65 g, 89% yield). 1H NMR (400 MHz, CDCl3) δ 8.64 (s, 1H), 8.40 (dd, J = 17.5, 5.6 Hz, 2H), 7.79 (br s, 1H), 2.29 (s, 3H).
References:
Blaazer, Antoni R.;Lange, Jos H.M.;Van Der Neut, Martina A.W.;Mulder, Arie;Den Boon, Femke S.;Werkman, Taco R.;Kruse, Chris G.;Wadman, Wytse J. [European Journal of Medicinal Chemistry,2011,vol. 46,# 10,p. 5086 - 5098] Location in patent:experimental part

13534-98-0
259 suppliers
$6.00/250mg

108-24-7
5 suppliers
$14.00/250ML

13535-03-0
4 suppliers
inquiry
5221-42-1
62 suppliers
$60.00/100mg

13535-03-0
4 suppliers
inquiry
1678-49-5
132 suppliers
$31.00/1g

13535-03-0
4 suppliers
inquiry