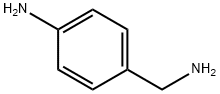
4-Aminobenzylamine synthesis
- Product Name:4-Aminobenzylamine
- CAS Number:4403-71-8
- Molecular formula:C7H10N2
- Molecular Weight:122.17
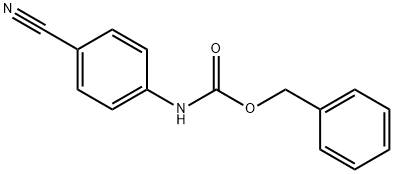
849042-08-6
1 suppliers
inquiry

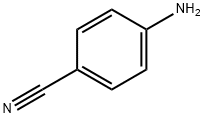
4403-71-8
155 suppliers
$77.70/10g
Yield:4403-71-8 72%
Reaction Conditions:
with methanol;sodium tetrahydroborate;nickel(II) chloride hexahydrate at 20; for 0.25 h;chemoselective reaction;
Steps:
General procedure
General procedure: In a typical experiment, benzyl 4-methoxyphenyl carbamate (1a, 1 mmol) and 10 cm3 methanol were placed in a 100 cm3 round-bottomed flask fitted with a water condenser and placed over a magnetic stirrer. Nickel(II) chloride hexahydrate (5 mmol) was added to the flask, followed by slow addition of sodium borohydride (15 mmol) with vigorous stirring. A vigorous reaction took place and the reaction mixture turned black due to in situ formation of nickel boride. The progress of the reaction was monitored by TLC (petroleum ether: ethyl acetate 80:20, v/v). After completion, the reaction mixture was filtered through a Celite pad (~2.5 cm) and washed with methanol (3x10 cm3). The solution was concentrated ona rotavapor and diluted with water (~50 cm3), followed by extraction with dichloromethane (3x10 cm3). The combined dichloromethane extract was washed with water and dried over anhyd. K2CO3. The solvent was removed ona rotary evaporator and the product was dried. 4-Anisidine(2a) was obtained as colourless solid in 88% yield. The products were identified by m.p., IR, and NMR spectra. The products Scheme 1, entry 24 and Scheme 2, entry 8 were purified by flash column chromatography on silica gel using petroleum ether:ethyl acetate (95:5, v/v) as eluent.
References:
Saroha, Mohit;Aggarwal, Komal;Bartwal, Gaurav;Khurana, Jitender M. [Monatshefte fur Chemie,2018,vol. 149,# 12,p. 2231 - 2235]
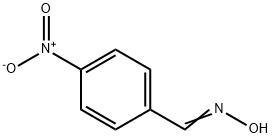
873-74-5
615 suppliers
$9.00/1g

4403-71-8
155 suppliers
$77.70/10g
1129-37-9
89 suppliers
$18.00/1g

4403-71-8
155 suppliers
$77.70/10g
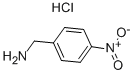
18600-42-5
142 suppliers
$9.00/250mg

4403-71-8
155 suppliers
$77.70/10g
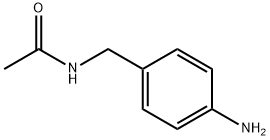
99362-10-4
15 suppliers
inquiry

4403-71-8
155 suppliers
$77.70/10g