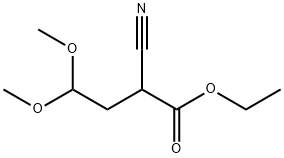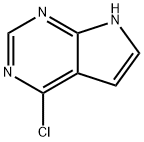
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine synthesis
- Product Name:4-Chloro-7H-pyrrolo[2,3-d]pyrimidine
- CAS Number:3680-69-1
- Molecular formula:C6H4ClN3
- Molecular Weight:153.57
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/NewsImg/2023-10-18/6383323147119370063141800.jpg)
![Pyrrolo[2,3-d]pyrimidin-4-ol](/CAS/GIF/3680-71-5.gif)
3680-71-5
401 suppliers
$6.00/1g
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
785 suppliers
$6.00/1g
Yield:3680-69-1 95%
Reaction Conditions:
with chlorine in 1-methyl-pyrrolidin-2-one;toluene at 160 - 180;Temperature;
Steps:
1-3; 1; 2 Small test:
Add 30ml of NMP / toluene mixed solvent with a volume ratio of 1/3 to a 100ml three-necked flask, add 1g of 4-hydroxypyrrolo [2,3-d] pyrimidine at room temperature, add 3ml of 1,2,3-trichloropropane, the gas in the bottle was replaced with chlorine gas, the temperature was increased to 160-180 ° C, and the reaction was stirred and refluxed for 4-5 hours. Negative pressure is used to remove excess solvent to obtain an oil. Start stirring and add 30ml of 0.5mol / L sodium hydroxide solution to the oil. Stir until the oil is dissolved and the solids are precipitated. / L sodium hydroxide solution was washed 3 times to obtain 1.08 g of 4-chloropyrrolopyrimidine product with a yield of 95% and a purity of 99.5%. The hydrogen spectrum and carbon spectrum were in accordance with the product structure.
References:
CN110790768,2020,A Location in patent:Paragraph 0019; 0021-0025; 0029-0040
![Pyrrolo[2,3-d]pyrimidin-4-ol](/CAS/GIF/3680-71-5.gif)
3680-71-5
401 suppliers
$6.00/1g
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
785 suppliers
$6.00/1g
![Pyrrolo[2,3-d]pyrimidin-4-ol](/CAS/GIF/3680-71-5.gif)
3680-71-5
401 suppliers
$6.00/1g
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
785 suppliers
$6.00/1g
16019-33-3
158 suppliers
$8.00/250mg
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
785 suppliers
$6.00/1g