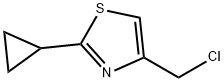
4-(chloromethyl)-2-cyclopropyl-1,3-thiazole synthesis
- Product Name:4-(chloromethyl)-2-cyclopropyl-1,3-thiazole
- CAS Number:757155-82-1
- Molecular formula:C7H8ClNS
- Molecular Weight:173.66
Yield:757155-82-1 79%
Reaction Conditions:
in acetone; for 8 h;Reflux;
Steps:
12.1
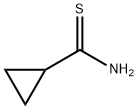
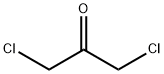
Step 1 : 4-(Chloromethyl)-2-cyclopropylthiazole[0174] To a slurry of cyclopropanecarboxamide (10 g, 0.12 mol) in MTBE (150 rnL) was charged P2S5 (5 g, 12 mmol). The mixture was heated to 100 0C for 2 h (monitored by TLC, EtOAc/hexane 1 :1) and cooled to room temperature. Supernatant was decanted and concentrated to afford the intermediate thioamide (6 g, 56%) as a light yellow solid. MS (ES) m/z 102.1 (M+ H+)). This was suspended in acetone (100 mL) and charged with 1,3- dichloroacetone (7.0 g, 0.055 mol). The mixture was heated to reflux for 8 h (monitored by TLC, EtOAc/hexane 1 :1), cooled to room temperature and concentrated. The residue was purified by silica gel chromatography using 2 to 10% EtOAc in hexane to afford the title compound (8.0 g, 79%) as a light brown oil. 1H NMR (400 MHz, CDCl3) δ 7.02 (s, IH), 4.62 (s, 2H), 2.32 (m, IH), 1.16 (m, 2H), 1.05 (m, 2H). MS (ES) m/z 174.1 (M+ H+).
References:
WO2010/54006,2010,A1 Location in patent:Page/Page column 65