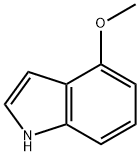
4-Methoxyindole synthesis
- Product Name:4-Methoxyindole
- CAS Number:4837-90-5
- Molecular formula:C9H9NO
- Molecular Weight:147.17
Stpe 2 : synthesis of 4-Methoxyindole, A suspension of 150 ml of zinc powder in 500 ml of 0.5N HCl is stirred for 1 hour at room temperature. The suspension is ded by suction, washed with water to neutral pH, with anhydrous EtOH and then with ether, and dried. To a solution of 10 g of the compound from the preceding step in 46 ml of acetic acid are added, portionwise, 31.6 g of activated zinc, while keeping the temperature between 20 and 30°C using an ice bath. The reaction mixture is stirred at room temperature for 30 minutes, and is filtered. The filtrate is extracted with EtOAc, the organic phase is washed with NaHCO3 solution and with saturated NaCl solution, and dried over MgSO4 , and the solvent is evaporated off under vacuum. The residue is chromatographed on silica gel, eluting with a cyclohexane/EtOAc mixture in a gradient of from (98/2 v/v) to (95/5 v/v). 4-Methoxy- 1H-indole; yield 1.6 g.
103260-65-7
213 suppliers
$8.00/100mg

4837-90-5
307 suppliers
$14.00/1g
Yield:4837-90-5 94%
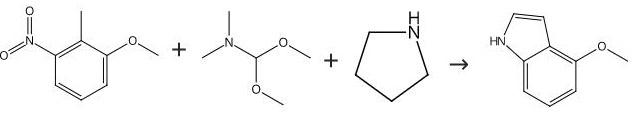
Reaction Conditions:
copper in quinoline for 2 h;Heating / reflux;
Steps:
1.1 (4-1)
(4-1) Production of 4-methoxy-1H-indole (5a) The carboxylic acid (4a) (3.65 g, 19.09 mmol, 1 eq), copper powder (849 mg, 13.36 mmol, 0.7 eq) obtained in (3-1) and freshly distilled quinoline (50mL) wererefluxedfor2hours. Themixture was then cooled, and filtered through a celite filter. The filtrate was poured into ice, and the solution was adjusted to pH 4 with conc. hydrochloric acid, and the solution was extracted three times with ethyl acetate (100 mL). The extracts were combined and washed three times with 2M hydrochloric acid (100 mL), further with saturated sodium hydrogen carbonate, and still further with aqueous solution of sodium chloride. The organic solution was dried over magnesium sulfate, and concentrated. The resulting concentrate was subjected to silica gel flush chromatography using hexane-ethyl acetate (85-15) to give white solid of the title compound (5a) (2.64 g, yield 94%).
References:
Meiji Dairies Corporation EP1533299, 2005, A1 Location in patent:Page/Page column 9
2380-94-1
465 suppliers
$8.00/250mg

74-88-4
339 suppliers
$15.00/10g

4837-90-5
307 suppliers
$14.00/1g
![1H-Indole, 4-methoxy-1-[(4-methylphenyl)sulfonyl]-](/CAS/20210305/GIF/112970-67-9.gif)
112970-67-9
2 suppliers
inquiry

4837-90-5
307 suppliers
$14.00/1g
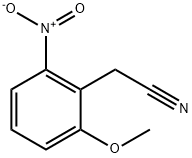
20876-27-1
29 suppliers
inquiry

4837-90-5
307 suppliers
$14.00/1g
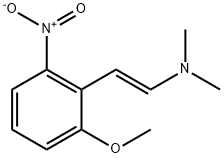
96631-91-3
0 suppliers
inquiry

4837-90-5
307 suppliers
$14.00/1g