
4-METHYL-5-PYRIDIN-2-YL-4H-1,2,4-TRIAZOLE-3-THIOL synthesis
- Product Name:4-METHYL-5-PYRIDIN-2-YL-4H-1,2,4-TRIAZOLE-3-THIOL
- CAS Number:34955-23-2
- Molecular formula:C8H8N4S
- Molecular Weight:192.24
![2-Pyridinecarboxylic acid, 2-[(methylamino)thioxomethyl]hydrazide](/CAS/20210305/GIF/34955-22-1.gif)
34955-22-1
0 suppliers
inquiry

34955-23-2
12 suppliers
$65.00/100mg
Yield:34955-23-2 157 mg
Reaction Conditions:
with sodium hydroxide in water at 70; for 16 h;
Steps:
6.A
2-Pyridylcarboxylic acid (123 mg, 1 eq) and 4-methyl-3-thiosemicarbazide (1 16 mg, 1 .1 eq) were suspended in 2 ml of DMF and the mixture was cooled to 0°C with an ice bath. T3P (50% DMF solution, 893 μΙ_, 1 .5 eq) and diisopropylethylamine (310 μΙ_, 1 .78 eq) were added slowly to the reaction mixture under stirring. The ice bath was removed and the mixture was reacted at room temperature for 16 hours. The complete conversion of the starting material was confirmed by HPLC. 2 ml of ethyl acetate, 2 ml of water and 2 ml of 4M NaOH aqueous solution were added to the reaction mixture. The phases were separated, and the organic layer was re-extracted with 4M NaOH aqueous solution. The combined aqueous phases were stirred 16 hours at 70°C. Conversion of the open intermediate into the desired product was confirmed by LC-MS. The reaction mixture pH was adjusted to 5 by dropwise addition of cone, hydrochloric acid under stirring. The precipitate was collected by filtration. 157 mg of product was obtained, which was used in the following step without any further purification.
References:
WO2018/189340,2018,A1 Location in patent:Page/Page column 61; 62
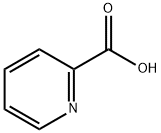
98-98-6
599 suppliers
$5.00/10g
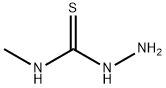
6610-29-3
206 suppliers
$19.00/1g

34955-23-2
12 suppliers
$65.00/100mg

6610-29-3
206 suppliers
$19.00/1g

34955-23-2
12 suppliers
$65.00/100mg

98-98-6
599 suppliers
$5.00/10g

34955-23-2
12 suppliers
$65.00/100mg