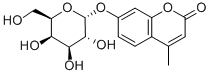
4-METHYLUMBELLIFERYL-ALPHA-D-GALACTOPYRANOSIDE synthesis
- Product Name:4-METHYLUMBELLIFERYL-ALPHA-D-GALACTOPYRANOSIDE
- CAS Number:38597-12-5
- Molecular formula:C16H18O8
- Molecular Weight:338.31
![4-Methyl-7-[(2,3,4,6-tetra-O-acetyl-α-D-galactopyranosyl)oxy]-2H-1-benzopyran-2-on](/CAS/20211123/GIF/67909-26-6.gif)
67909-26-6
0 suppliers
inquiry

38597-12-5
124 suppliers
$25.00/10mg
Yield: 80%
Reaction Conditions:
with potassium hydroxide in tetrahydrofuran at 20; for 0.25 h;
Steps:
4-Methylumbelliferyl α-D-galactopyranoside (4c)
Compound 3c (400 mg, 0.79 mmol) was O-deacetylated for 15 min at room temperature with KOH (1.0 M in anhydrous MeOH; 237 μL, 0.3 equiv.) in dry tetrahydrofuran (8 mL) and anhydrous MeOH (4 mL). The white solid precipitate was filtered off,washed with ethanol until the washings were no longer fluorescent, and dried. White powder; yield: 214 mg (80%); mp: 222-223 °C; [α]20D +238° (c = 0.30, H2O); {lit. [27] mp: 212-217 °C; [α]20D +135° (c = 1.37,pyridine)}. 1H-NMR (500 MHz, DMSO-d6): δ = 7.70 (d, J5′,6′ = 9.0 Hz, 1H, H-5′), 7.09-7.07 (m, 2H, H-6′,H-8′), 6.24 (d, J3′,Me′ = 1.0 Hz, 1H, H-3′), 5.60 (d, J1,2 = 3.0 Hz, 1H, H-1), 4.99 (d, J = 6.5 Hz, 1H), 4.79 (d,J = 5.0 Hz, 1H), 4.61 (d, J = 4.5 Hz, 1H), 4.53 (t, J = 5.7 Hz, 1H), 3.83-3.74 (m, 3H), 3.64 (t, J = 5.7 Hz, 1H,H-5), 3.52 (dt, J = 11.0, 6.2 Hz, 1H, H-6a), 3.38 (dt, J = 11.0, 6.2 Hz, 1H, H-6b), 2.40 (s, 3H, H-Me′).13C-NMR (75 MHz, DMSO-d6): δ = 160.07 (C-2′), 160.07 (C-7′), 154.30, 153.32, 126.32, 113.92, 113.82, 111.56(C-3′, C-4′, C-5′, C-6′, C-4a′, C-8a′), 103.65 (C-8′), 98.07 (C-1), 72.68, 69.30, 68.42, 67.71, 60.20 (C-2, C-3,C-4, C-5, C-6), 18.09 (C-Me′). HRMS (ESI): m/z [M + Na]+ calcd for C16H18NaO8: 361.0894; found: 361.0897. 1H- and 13C-NMR, and HRMS spectrograms are seen in the Supplement Materials.
References:
Wei, Xianhu;Ma, Yanxia;Wu, Qingping;Zhang, Jumei;Cai, Zhihe;Lu, Mianfei;Ferro, Vito [Molecules,2015,vol. 20,# 12,p. 21681 - 21699]
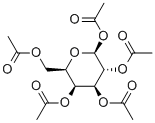
4163-60-4
343 suppliers
$6.00/5g

38597-12-5
124 suppliers
$25.00/10mg