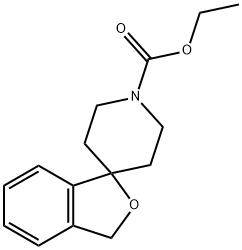
ethyl 3H-spiro[isobenzofuran-1,4'-piperidine]-1'-carboxylate synthesis
- Product Name:ethyl 3H-spiro[isobenzofuran-1,4'-piperidine]-1'-carboxylate
- CAS Number:42191-83-3
- Molecular formula:C15H19NO3
- Molecular Weight:261.32
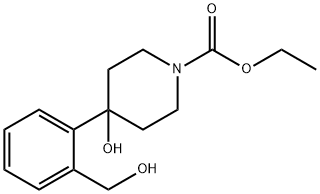
71546-51-5
23 suppliers
$248.88/5g
![ethyl 3H-spiro[isobenzofuran-1,4'-piperidine]-1'-carboxylate](/CAS/GIF/42191-83-3.gif)
42191-83-3
26 suppliers
$316.00/5g
Yield:42191-83-3 100%
Reaction Conditions:
with methanesulfonyl chloride;triethylamine in toluene at 60; for 0.5 h;
Steps:
2 [EXANTPLE] 2: synthesis of 1'-carbethoxy-spiro [isobenzofuran-1-(3H),4'-piperidine] (2) through ring closure
[EXANTPLE] 2: synthesis of 1'-carbethoxy-spiro [isobenzofuran-1-(3H),4'-piperidine] (2) through ring closure. Crude [L-CARBETHOXY-4-HYDROXY-4-(2-HYDROXYMETHYLPHENYL)-PIPERIDINE (T)] prepared according to example 1 (4.5 kg) is dissolved in toluene (27.4 kg). Triethyl amine (3.5 kg) is added. A solution [OF METHANESULFONYL] chloride (1.25 kg) in toluene (12 kg) is prepared, and the mixture is added at a rate that keeps the temperature of the reaction below [60 °C.] When the addition is complete, the reaction is stirred for 30 minutes. The reaction is checked by HPLC or GC. If completed, water (40 kg) is added to the reaction mixture. The organic phase is separated after thorough mixing and the solvent is evaporated. Yield of crude [L'-CARBETHOXY-SPIRO] [isobenzofuran-1 (3H), 4'-piperidine] as an oil 4.65 kg (>100%).
References:
WO2004/26855,2004,A1 Location in patent:Page 10-11
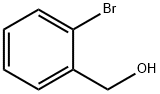
18982-54-2
277 suppliers
$5.00/5g
![ethyl 3H-spiro[isobenzofuran-1,4'-piperidine]-1'-carboxylate](/CAS/GIF/42191-83-3.gif)
42191-83-3
26 suppliers
$316.00/5g
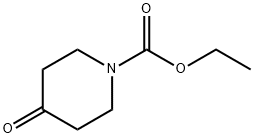
29976-53-2
405 suppliers
$5.00/5g
![ethyl 3H-spiro[isobenzofuran-1,4'-piperidine]-1'-carboxylate](/CAS/GIF/42191-83-3.gif)
42191-83-3
26 suppliers
$316.00/5g