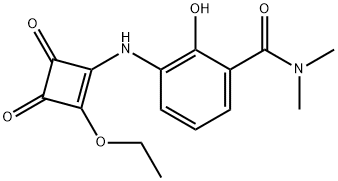
3-[(2-ETHOXY-3,4-DIOXO-1-CYCLOBUTEN-1-YL)AMINO]-2-HYDROXY-N,N-DIMETHYL-BENZAMIDE synthesis
- Product Name:3-[(2-ETHOXY-3,4-DIOXO-1-CYCLOBUTEN-1-YL)AMINO]-2-HYDROXY-N,N-DIMETHYL-BENZAMIDE
- CAS Number:464913-33-5
- Molecular formula:C15H16N2O5
- Molecular Weight:304.3
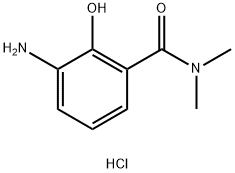
1000993-70-3
26 suppliers
inquiry
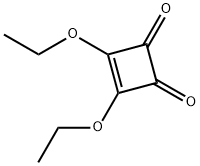
5231-87-8
215 suppliers
$10.00/1g
![3-[(2-ETHOXY-3,4-DIOXO-1-CYCLOBUTEN-1-YL)AMINO]-2-HYDROXY-N,N-DIMETHYL-BENZAMIDE](/CAS/GIF/464913-33-5.gif)
464913-33-5
16 suppliers
inquiry
Yield:464913-33-5 88%
Reaction Conditions:
Stage #1: 2-hydroxy-N,N-dimethyl-3-amino-benzamide hydrochloride;3,4-diethoxy-3-cyclobuten-1,2-dionewith triethylamine in ethanol at 0 - 10; for 10 h;
Stage #2: with acetic acid in ethanol at 0; for 0.25 h;Product distribution / selectivity;Heating / reflux;
Steps:
7
Preparative Example 7; Preparation of Compound (209B); From Diethylsquarate (216) and Compound(213)Charged 44.0 kg of the compound (213), 225 kg dry ethanol and 41.8 kg of the compound (216) to a 300 gallon glass lined reactor equipped with a thermocouple, N2 inlet and feed bottle. Adjusted the batch to temperature between 0 and 10αC. Over about 1 hour, charged 17.1 kg triethylamine (TEA) to the batch while maintaining the batch at a temperature between 0 and 100C. After the addition of TEA was complete, agitated the batch for about three hours at a temperature between 0 and 100C. Over about 3 hours, charged additional 8.2 kg triethylamine (TEA) to the batch while maintaining the batch at a temperature between 0 and 100C. After the addition of TEA was complete, agitated the batch for about three hours at a temperature between 0 and 1O0C. Charged 19 liters acetic acid while maintaining the batch at a temperature between 0 and 100C. Adjusted the batch volume to 440 liters by adding dry ethanol. Heated the batch to reflux and maintained for about 15 minutes. Adjusted the temperature to about 0 to 10°C over about 2 hours. Filtered the batch and washed the filter cake with 220 liters 50 % v/v ethanol in water. Dried the batch in a vacuum oven for at least 12 hours at 50 to 600C. Yield 52 kg , 88 %.1HNMR (CD3CN) 7.61 (1H, d); 7.28 (1H, d); 6.96 (1 H, t); 4.69 (2H, q); 3.10 (6H, s), 1.44 (3H1 1).
References:
WO2008/5570,2008,A1 Location in patent:Page/Page column 76
85-38-1
249 suppliers
$12.00/1g
![3-[(2-ETHOXY-3,4-DIOXO-1-CYCLOBUTEN-1-YL)AMINO]-2-HYDROXY-N,N-DIMETHYL-BENZAMIDE](/CAS/GIF/464913-33-5.gif)
464913-33-5
16 suppliers
inquiry

66952-65-6
11 suppliers
inquiry
![3-[(2-ETHOXY-3,4-DIOXO-1-CYCLOBUTEN-1-YL)AMINO]-2-HYDROXY-N,N-DIMETHYL-BENZAMIDE](/CAS/GIF/464913-33-5.gif)
464913-33-5
16 suppliers
inquiry

