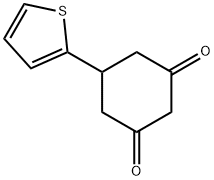
5-(2-Thienyl)-1,3-cyclohexanedione synthesis
- Product Name:5-(2-Thienyl)-1,3-cyclohexanedione
- CAS Number:23994-65-2
- Molecular formula:C10H10O2S
- Molecular Weight:194.25
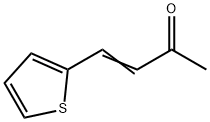
874-83-9
54 suppliers
$18.00/1g

105-53-3
728 suppliers
$5.00/25g

23994-65-2
20 suppliers
$258.01/1gm:
Yield:23994-65-2 69%
Reaction Conditions:
Stage #1: 2-thenylideneacetone;diethyl malonatewith sodium in methanol at 80; for 4 h;
Stage #2: with sodium hydroxide in methanol;water at 80; for 0.666667 h;
Stage #3: with hydrogenchloride in methanol;water;Reflux;
Steps:
I
[0069] Sodium metal (1 eq., 970 mg) was dissolved in dry MeOH (20 ml). After 0.5 hours, diethylmalonate (1 eq., 6.2 ml) was added dropwise to maintain temperature between 15-20 °C; (8) is then added at 60 °C. The reaction mixture was refluxed (80 °C) for 4 hours and made alkaline with aqueous NaOH (13 ml of 17.6 grams NaOH in 70 ml H20). The alkaline solution was heated at 80 °C for 40 minutes, and then cone. HC1 (13 ml) was added under reflux. On cooling, a yellow solid (9) was obtained (5.3 grams, 69%> yield).
References:
WO2011/62964,2011,A1 Location in patent:Page/Page column 19
71637-34-8
220 suppliers
$5.00/1g

23994-65-2
20 suppliers
$258.01/1gm:
98-03-3
523 suppliers
$5.00/25g

23994-65-2
20 suppliers
$258.01/1gm: