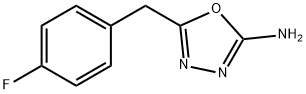
5-(4-fluorobenzyl)-1,3,4-oxadiazol-2-amine synthesis
- Product Name:5-(4-fluorobenzyl)-1,3,4-oxadiazol-2-amine
- CAS Number:828911-26-8
- Molecular formula:C9H8FN3O
- Molecular Weight:193.18
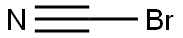
506-68-3
146 suppliers
$10.00/1g
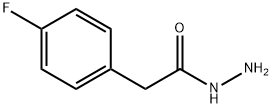
34547-28-9
81 suppliers
$9.00/250mg

828911-26-8
20 suppliers
$45.00/25mg
Yield:828911-26-8 91.5%
Reaction Conditions:
with potassium hydrogencarbonate in methanol at 0 - 20; for 19 h;
Steps:
Solid BrCN 13.37g (130 mmol, 1.1 eq. ) was added in one portion into ice-cooled slurry of (4- Fluoro-phenyl)-acetic acid hydrazide (19. 85G, 118 MMOL) and KHC03 14.77g (147. 5 mmol, 1.25 eq) in MEOH (150ML) in a 1 L flask. (Followed by MEOH 10 mL to wash the funnel). The mixture was stirred on ice bath at 0-5 °C for 2 hours in a loose-capped flask, then the bath allowed to melt gradually and then the mixture was stirred at 5 to 20°C overnight (17 hrs). The reaction mixture was diluted with water (200mL), stirred for 1 hour in an open flask, then cooled on ice bath. The precipitate was collected by filtration, washed with water and dried on highvac. [1 FRACTION] The supernatants were concentrated on rotavap form warm (40 °C) water bath to remove all MEOH and some water. The obtained slurry was cooled to R. T. , the precipitate was collected by filtration, washed with water and dried on highvac. [2ND fraction]. Combined yield : 20. 836G (91. 5%) of a white cryst. SOLID. RH-NMR (DMSO-D6, 400 MHz): 7. 289 (m, 2H), 7.148 (m, 2H), 6. 873 (br s, 2H), 4. 014 (s, 2H) ; 9F-NMR (DMSO-D6, 376.5 MHz):- 116.01 (m, 1 F).
References:
WO2005/10005,2005,A1 Location in patent:Page/Page column 30