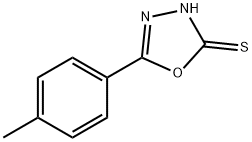
5-(4-METHYLPHENYL)-1 3 4-OXADIAZOLE-2-& synthesis
- Product Name:5-(4-METHYLPHENYL)-1 3 4-OXADIAZOLE-2-&
- CAS Number:31130-15-1
- Molecular formula:C9H8N2OS
- Molecular Weight:192.24
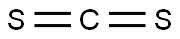
75-15-0
233 suppliers
$40.00/100g
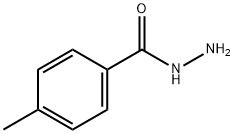
3619-22-5
111 suppliers
$12.00/5g

31130-15-1
33 suppliers
$67.00/5G
Yield:31130-15-1 86.7%
Reaction Conditions:
with potassium hydroxide in methanol; for 0.0666667 h;Microwave irradiation;
Steps:
General procedure for synthesis of substituted 1,3,4-oxadiazole-2-thiol:
General procedure: Required acid hydrazide (3a-h, 0.1 mol), carbondisulphide (0.12 mol), potassium hydroxide (0.1 mol) and250 ml methanol were mixed in a flask and refluxed in thefume cupboard30,31. Heating was continued till evolution ofhydrogen sulphide stopped (about 8-10 h) which was testedby passing the gas over a filter paper dipped in lead acetate.The excess of solvents were distilled off and then contentsdiluted with distilled water. The neutralization with dilute hydrochloricacid gave the product. Rectified spirit was usedfor recystallization and melting point was determined(Table 3).Microwave method: The same procedure as stated abovewas adopted using 140 ml methanol in a microwave reactorat 280 Watt (power) for 3-4 min. Separation of precipitatewas done as in conventional method. The product was recrystallizedfrom rectified spirit (Table 3
References:
Upadhyay, Prabhat Kumar;Mishra, Pradeep [Journal of the Indian Chemical Society,2018,vol. 95,# 6,p. 661 - 666]

38499-94-4
0 suppliers
inquiry

31130-15-1
33 suppliers
$67.00/5G
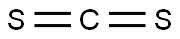
75-15-0
233 suppliers
$40.00/100g
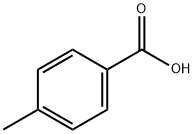
99-94-5
569 suppliers
$9.00/1g

31130-15-1
33 suppliers
$67.00/5G
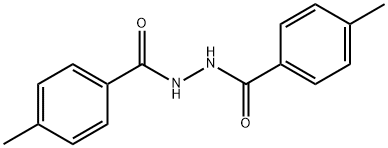
1530-73-0
20 suppliers
$42.39/1g
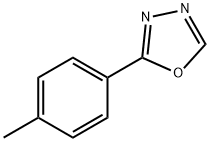
827-58-7
29 suppliers
$123.70/1gm:

31130-15-1
33 suppliers
$67.00/5G
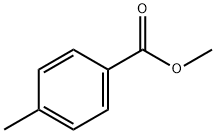
99-75-2
269 suppliers
$6.00/10g

31130-15-1
33 suppliers
$67.00/5G