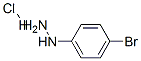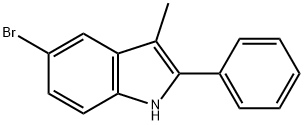
5-BroMo-3-Methyl-2-phenyl-1H-indole synthesis
- Product Name:5-BroMo-3-Methyl-2-phenyl-1H-indole
- CAS Number:848749-72-4
- Molecular formula:C15H12BrN
- Molecular Weight:286.17
Yield:848749-72-4 65%
Reaction Conditions:
in ethanol; for 18 h;Heating / reflux;
Steps:
3.1 Example 3; Synthesis of 1-Benzyl-3-methyl-2-phenyl-5-[4-(1H-tetrazol-5-ylmethoxy)-phenyl]-1H-indole; Step 1: Synthesis of 5-Bromo-3-methyl-2-phenyl-1H-indole.
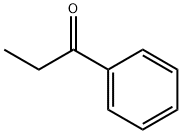
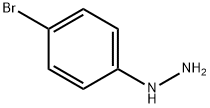
A mixture of 4-bromophenylhydrazine, hydrochloride (4.928 g, 22.05 mmol) and propriophenone (2.818 g, 21 mmol) in EtOH (60 ml) was refluxed under N2. After 18 hours, the reaction mixture was cooled and concentrated. The residue was partitioned between EtOAc (150 ml) and 1N HCl (30 ml). The layers were shaken, separated, and the organic layer washed with 1N HCl (2*20 ml), water (2*20 ml) and brine (2*20 ml), dried over Na2SO4, filtered, concentrated, and dried to give a solid (6.006 g). The solid was recrystallized from hexane to give the desired product (4.10 g, 14.33 mmol, 65%) as an off-white solid, mp 138-140° C. 1H NMR (DMSO-d6) δ 2.37 (s, 3H), 7.18-7.20 (m, 1H), 7.30 (d, J=8.4 Hz, 1H), 7.37 (t, J=7.0 Hz, 1H), 7.51 (t, J=7.6 Hz, 2H), 7.66 (d, J=8.1 Hz, 2H), 7.70 (s, 1H), 11.36 (s, 1H); [ESI(-)], m/z 284/286 (M-H)-.
References:
US2005/70592,2005,A1 Location in patent:Page/Page column 15

106-40-1
455 suppliers
$12.00/5g

848749-72-4
4 suppliers
inquiry
29124-56-9
118 suppliers
$18.00/1g

848749-72-4
4 suppliers
inquiry