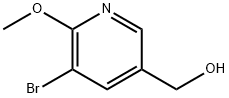
(5-bromo-6-methoxypyridin-3-yl)methanol synthesis
- Product Name:(5-bromo-6-methoxypyridin-3-yl)methanol
- CAS Number:1219936-55-6
- Molecular formula:C7H8BrNO2
- Molecular Weight:218.05
1186194-46-6
48 suppliers
$10.00/100mg

1219936-55-6
21 suppliers
inquiry
Yield:1219936-55-6 82%
Reaction Conditions:
Stage #1: 5-bromo-6-methoxypyridine-3-carboxylic acidwith 1,1'-carbonyldiimidazole in tetrahydrofuran at 20; for 1 h;
Stage #2: with sodium tetrahydroborate in tetrahydrofuran;water at 0; for 0.5 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;water at 20; for 0.5 h;
Steps:
Step 1: (5-bromo-6-methoxy-pyridin-3-yl)methanol
A solution of 5-bromo-6-methoxy-pyridine-3-carboxylic acid (300 mg, 1.29 mmol) in THF (3 mL) was treated with CDI (335 uL, 1.94 mmol). The mixture was stirred at rt for 1 h. A solution of NaBH4 (245 mg, 6.46 mmol) in H2O (3 ml) was added dropwise at 0°C and stirred at this temperature for 30 min. HCl 2M (3 mL) was added dropwise and the resulting mixture stirred for 30 minutes at rt. Mixture was poured into NaHCO3 saturated solution (6 mL) and extracted with EtOAc (3x30 mL). The organic layer was washed with H2O, brine, dried over Na2SO4, filtered, concentrated in vacuo and purified by flash chromatography (gradient elution 0-50% EtOAc in Petroleum Ether) to afford the title compound as a white solid (232 mg, 82%). LCMS (ES+) m/z 218-220 (M+H)+, RT 1.13 min.
References:
WO2021/28362,2021,A1 Location in patent:Page/Page column 152-153
93349-99-6
93 suppliers
$12.00/100mg

1219936-55-6
21 suppliers
inquiry