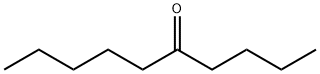
5-DECANONE synthesis
- Product Name:5-DECANONE
- CAS Number:820-29-1
- Molecular formula:C10H20O
- Molecular Weight:156.27

7433-56-9
44 suppliers
$36.50/1g

820-29-1
27 suppliers
inquiry
Yield: 86%
Reaction Conditions:
with oxygen;palladium dichloride in N,N-dimethyl acetamide;water at 80; under 4500.45 - 6750.68 Torr; for 10 h;Product distribution / selectivity;Autoclave;
Steps:
4
(Examples 2 to 6) Oxidation reactions were conducted in the same manner as in Example 1, except that trans-4-octene was replaced with trans-2-octene (112 mg, 1.0 mmol), trans-3-octene (112 mg, 1.0 mmol), trans-5-decene (140 mg, 1.0 mmol), 7-tetradecene (196 mg, 1.0 mmol), or trans-3-hexene (84 mg, 1.0 mmol) in the respective reactions. The products were analyzed in the same manner as in Example 1. As a result, it was found that an oxo group (=O) was bonded to a carbon atom in the C=C bond of each internal olefin, so that the corresponding ketone was formed. Table 2 shows the yield of the corresponding ketone based on the amount of each internal olefin fed, and the selectivity to the corresponding ketone with respect to the total amount of the product.; Example 1); Palladium chloride (8.8 mg, 0.05 mmol), dimethylacetamide (DMA, 5 ml), and water (0.5 ml) were placed in a pressure vessel, and heated to 80°Cto dissolve palladium chloride. The obtained solution was transferred to an autoclave reactor. Then, the pressure inside the reactor was raised to 0.9 MPa by supplying oxygen gas thereto, and stirring was conducted for 1 hour. The pressure inside the reactor was released, and trans-4-octene (112 mg, 1.0 mmol) was added thereto. Then, the pressure inside the reactor was raised to 0.6 MPa by supplying oxygen gas thereto, and the oxidation reaction was allowed to proceed at 80°C for 10 hours. After completion of the reaction, the product was analyzed by using a gas chromatograph equipped with a FID detector ("GC-2014" manufactured by Shimadzu Corporation, column: KOCL 3 m). As a result, it was found that an oxo group (=O) was bonded to a carbon atom in the C=C bond of trans-4-octene , so that 4-octanone was formed. Accordingly, trans-4-octene was presumably oxidized as shown in the following reaction the formula (I): [Show Image] Table 1 shows the yield of 4-octanone based on the amount of trans-4 -octene supplied, and the selectivity to 4-octanone with respect to the total amount of the product.
References:
JX Nippon Oil & Energy Corporation EP2364965, 2011, A1 Location in patent:Page/Page column 9-10

1942-46-7
93 suppliers
$6.00/250mg

820-29-1
27 suppliers
inquiry
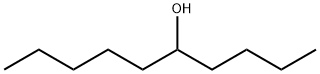
5205-34-5
52 suppliers
$60.00/100mg

820-29-1
27 suppliers
inquiry
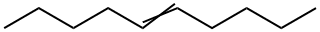
19689-19-1
4 suppliers
inquiry

820-29-1
27 suppliers
inquiry

