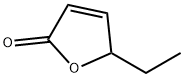
5-ethylfuran-2(5H)-one synthesis
- Product Name:5-ethylfuran-2(5H)-one
- CAS Number:2407-43-4
- Molecular formula:C6H8O2
- Molecular Weight:112.13
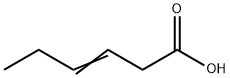
4219-24-3
23 suppliers
inquiry

2407-43-4
10 suppliers
inquiry
Yield: 93%
Reaction Conditions:
Stage #1:hex-3-enoic acid with Oxalyl bromide;dimethyl sulfoxide in dichloromethane at -10 - 20;Inert atmosphere;
Stage #2: with sodium hydroxide in dichloromethane;water for 5 h;Reagent/catalyst;
Steps:
General Procedure for synthesis of butenolides using DMSO and (COBr)2
General procedure: To a solution of oxalyl bromide (1.07 mL, 7.5 mmol, 1.5 equiv) in CH2Cl2 (10 mL) cooled at -10 °C was added dropwise a solution of dimethyl sulfoxide (0.53 mL, 7.5 mmol, 1.5 equiv) in CH2Cl2 (10 mL) under the atmosphere of nitrogen. After 10 min, a solution of 3-alkenoic acid (5 mmol, 1.0 equiv) in CH2Cl2 (10 mL) was added. The mixture was then allowed to warm up to room temperature and stirred for 1 h. Distilled water (20 mL) was added dropwise at 0 °C. After stirring for 10 min, NaOH (1.00 g, 25 mmol, 5.0 equiv) or K2CO3 (3.45 g, 25 mmol, 5.0 equiv) was added and stirred for 5 h. The organic layer was separated and successively washed with brine (2 × 30 mL). The combined organic extracts were dried (Na2SO4), filtered, and concentrated in vacuum to afford the corresponding γ-butenolides.
References:
Ding, Rui;Liu, Yongguo;Liu, Lei;Li, Huimin;Tao, Sichen;Sun, Baoguo;Tian, Hongyu [Synthetic Communications,2019,vol. 49,# 21,p. 3001 - 3007] Location in patent:supporting information
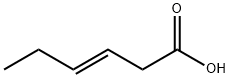
1577-18-0
169 suppliers
$11.00/5g

2407-43-4
10 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2407-43-4
10 suppliers
inquiry

100591-65-9
0 suppliers
inquiry

2407-43-4
10 suppliers
inquiry

84477-19-0
0 suppliers
inquiry

2407-43-4
10 suppliers
inquiry