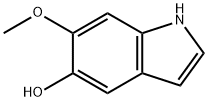
5-hydroxy-6-methoxyindole synthesis
- Product Name:5-hydroxy-6-methoxyindole
- CAS Number:2380-83-8
- Molecular formula:C9H9NO2
- Molecular Weight:163.17
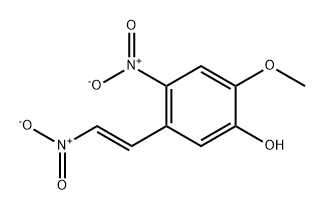
![1-methoxy-5-nitro-4-[(E)-2-nitroethenyl]-2-phenylmethoxybenzene](/CAS/20180529/GIF/98258-75-4.gif)
98258-75-4
0 suppliers
inquiry

2380-83-8
14 suppliers
inquiry
Yield:2380-83-8 46%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol;water; for 3 h;
Steps:
1.3 6-Methoxy-1H-indol-5-ol
Production Example 1-3
6-Methoxy-1H-indol-5-ol
(E)-1-(Benzyloxy)-2-methoxy-4-nitro-5-(2-nitrovinyl)benzene described in Production Example 1-2 (9.4 g, 28.5 mmol) was suspended in methanol (300 mL), then 10% palladium-carbon (water content, 50%) (3.09 g) was added, and the mixture was stirred under hydrogen atmosphere for 3 hours.
The catalyst was filtered off with celite and washed with methanol.
The filtrate was concentrated under vacuum and the resultant was purified with silica gel column chromatography (n-heptane:ethyl acetate=3:1-3:7).
The target fraction was concentrated under vacuum to obtain the title compound (2.12 g, 46%).
1H-NMR Spectrum (CDCl3) δ (ppm): 3.92 (3H, s), 5.45 (1H, s), 6.39-6.44 (1H, m), 6.88 (1H, s), 7.08 (1H, t, J=2.9 Hz), 7.14 (1H, s), 7.95 (1H, brs).
References:
US2014/235614,2014,A1 Location in patent:Paragraph 0258; 0259; 0260
98258-77-6
0 suppliers
inquiry

2380-83-8
14 suppliers
inquiry
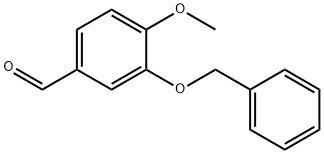
6346-05-0
214 suppliers
$7.00/1g

2380-83-8
14 suppliers
inquiry
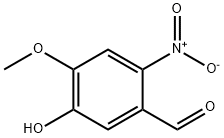
58749-47-6
48 suppliers
inquiry

2380-83-8
14 suppliers
inquiry
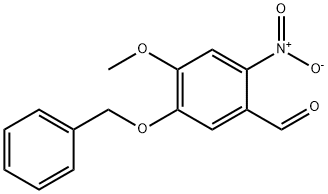
58662-50-3
22 suppliers
inquiry

2380-83-8
14 suppliers
inquiry