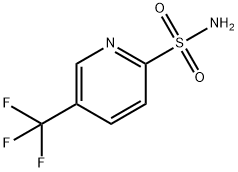
5-(trifluoroMethyl)pyridine-2-sulfonaMide synthesis
- Product Name:5-(trifluoroMethyl)pyridine-2-sulfonaMide
- CAS Number:332366-24-2
- Molecular formula:C6H5F3N2O2S
- Molecular Weight:226.18
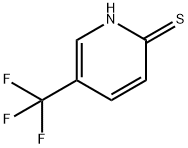
76041-72-0
137 suppliers
$8.00/1g

332366-24-2
24 suppliers
$265.00/100mg
Yield:332366-24-2 10%
Reaction Conditions:
Stage #1: 5-trifluoromethylpyridine-2(1H)-thionewith chlorine in water;acetic acid at 0; for 0.75 - 1 h;
Stage #2: with ammonium hydroxide in water;acetic acid at 0; for 0.166667 h;
Steps:
20 5-(Trifluoromethyl)pyridine-2-sulfonamide (117)
5-(Trifluoromethylpyridin-2(1H-thione (116), 10.0 g, 55.8 mmol) was placed in a flask containing 100 mL of water and 50 mL of acetic acid. The mixture was cooled to 0° C. and chlorine gas was bubbled into the mixture for 45-60 minutes. Water (100 mL) was added and a precipitate formed which was collected by filtration. This solid was cooled in an ice bath and in a separate flask 100 mL of cone. NH4OH was also cooled in an ice bath. After 10 minutes, the cooled NH4OH solution was added to the solid and the mixture was stirred overnight at room temperature. The mixture was then extracted with Et2O (100 mL). The aqueous layer was collected and concentrated to give crude product that was purified by column chromatography on silica gel using EtOAc/hexanes gradients to afford 1.30 g of (117) (10%) as an off-white solid.
References:
US2008/21056,2008,A1 Location in patent:Page/Page column 93