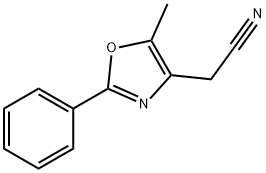
524959-64-6 synthesis
- Product Name:524959-64-6
- CAS Number:524959-64-6
- Molecular formula:C12H10N2O
- Molecular Weight:198.22
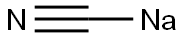
143-33-9
5 suppliers
$10.00/1g

103788-61-0
50 suppliers
$149.00/250mg

524959-64-6
7 suppliers
inquiry
Yield:524959-64-6 92%
Reaction Conditions:
methyl tributylammonium chloride in water;toluene at 80 - 85; for 6 h;
Steps:
1.b
) Reaction of the oxazole chloride1634 g (7.87 mol) of oxazole chloride, 463 g (9.44 mol) of NaCN, 28 g (0.119 mol) of tributylmethylammonium chloride, 4.9 1 of toluene and 0.24 1 of H2O were introduced into the reactor, and this mixture was heated at 80-85°C while stirring vigorously for 6 h. After the reaction mixture had cooled to 200C, dilute aqueous Na2CO3 solution was added and, after phase separation, the organic phase was extracted twice with dilute aqueous Na2CO3 solution. The residue after concentration of the organic phase in vacuo was dissolved in diisopropyl ether, filtered through activated carbon and crystallized. 1435 g (7.24 mol, 92%) of the oxazole nitrile were obtained.
References:
WO2006/108491,2006,A1 Location in patent:Page/Page column 7
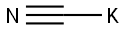
151-50-8
4 suppliers
$43.36/100g

103788-61-0
50 suppliers
$149.00/250mg

524959-64-6
7 suppliers
inquiry
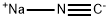
773837-37-9
0 suppliers
inquiry

103788-61-0
50 suppliers
$149.00/250mg

524959-64-6
7 suppliers
inquiry

27492-46-2
1 suppliers
inquiry

524959-64-6
7 suppliers
inquiry

100-52-7
948 suppliers
$20.37/250g

524959-64-6
7 suppliers
inquiry