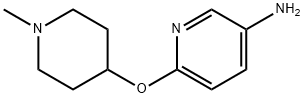
6-[(1-methylpiperidin-4-yl)oxy]pyridin-3-amine synthesis
- Product Name:6-[(1-methylpiperidin-4-yl)oxy]pyridin-3-amine
- CAS Number:944401-79-0
- Molecular formula:C11H17N3O
- Molecular Weight:207.27
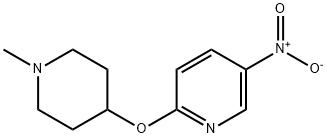
944401-78-9
2 suppliers
inquiry
![6-[(1-methylpiperidin-4-yl)oxy]pyridin-3-amine](/CAS/20180529/GIF/944401-79-0.gif)
944401-79-0
9 suppliers
inquiry
Yield:944401-79-0 860 mg
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 35; under 760.051 Torr;
Steps:
22.2 Step 22.2: 6-(1 -Methylpiperidin-4-yloxy)pyridin-3-ylamine
Step 22.2: 6-(1 -Methylpiperidin-4-yloxy)pyridin-3-ylamine 1.05 g of the compound obtained in step 20.1 are dissolved in 90 ml of MeOH and then hydrogenated at atmospheric pressure at 35°C over 10% Pd/C. The medium is filtered and then evaporated under reduced pressure to give 860 mg of 6-(1 - methylpiperidin-4-yloxy)pyridin-3-ylamine.
References:
WO2013/111106,2013,A1 Location in patent:Page/Page column 109
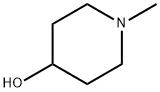
106-52-5
363 suppliers
$6.00/25g
![6-[(1-methylpiperidin-4-yl)oxy]pyridin-3-amine](/CAS/20180529/GIF/944401-79-0.gif)
944401-79-0
9 suppliers
inquiry
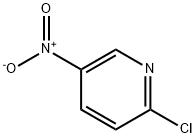
4548-45-2
530 suppliers
$6.00/5g
![6-[(1-methylpiperidin-4-yl)oxy]pyridin-3-amine](/CAS/20180529/GIF/944401-79-0.gif)
944401-79-0
9 suppliers
inquiry