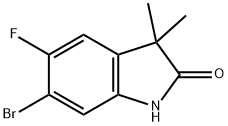
6-bromo-5-fluoro-3,3-dimethylindolin-2-one synthesis
- Product Name:6-bromo-5-fluoro-3,3-dimethylindolin-2-one
- CAS Number:1379313-54-8
- Molecular formula:C10H9BrFNO
- Molecular Weight:258.09
893620-44-5
58 suppliers
$76.00/100mg

74-88-4
342 suppliers
$15.00/10g

1379313-54-8
6 suppliers
inquiry
Yield:1379313-54-8 3.6 g
Reaction Conditions:
Stage #1: 6-bromo-5-fluoroindolin-2-onewith copper(I) bromide dimethylsulfide complex;potassium tert-butylate in tetrahydrofuran;Cooling with ice;
Stage #2: methyl iodide in tetrahydrofuran at 2 - 20; for 16.5 h;
Steps:
36.a Example 36 N-(l-cyclopropyl-5-fluoro-3 3-dimethyl-2-oxoindolin-6-yl)isonicotinamide
a) 6-Bromo-5-fluoro-3,3-dimethylindolin-2-one To a solution of potassium tert-butoxide (9.27 g, 82.6 mmol) in dry THF (50 ml) under icebath cooling was added 6-bromo-5-fluoroindolin-2-one (example 20c, 3.8 g, 16.5 mmol) in portions, followed by copper (I) bromide-dimethyl sulfide complex (340 mg, 1.65 mmol). After cooling to 2°C methyl iodide (4.92 g, 2.17 ml, 34.7 mmol) was added slowly over a period of 30 minutes. The reaction mixture was warmed to room temperature, stirred for 16 hours, then cooled to 0°C and carefully quenched with saturated ammonium chloride solution. The mixture was diluted with tert-butyl methyl ether and water. The aqueous phase was extracted with tert-butyl methyl ether, the combined organic phases were dried over sodium sulfate, the solvent was evaporated and the residue purified by silica gel chromatography using ethyl acetate/ heptane as eluent and followed by trituration with diethyl ether. The title compound was obtained as yellow solid (3.6 g). MS ESI (m/z): 258.0/ 260.0 [(M+H)+]. 1H NMR (CDCI3, 400 MHz): (ppm) = 10.44 (bs, 1H), 7.48-7.45 (m, 1H), 7.05-7.04 (m, 1H), 1.25 (s, 6H).
References:
WO2014/40969,2014,A1 Location in patent:Page/Page column 38