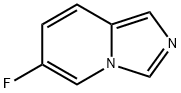
6-fluoroimidazo[1,5-a]pyridine synthesis
- Product Name:6-fluoroimidazo[1,5-a]pyridine
- CAS Number:1426421-17-1
- Molecular formula:C7H5FN2
- Molecular Weight:136.13
![Formamide, N-[(5-fluoro-2-pyridinyl)methyl]-](/CAS/20210305/GIF/1426421-16-0.gif)
1426421-16-0
0 suppliers
inquiry
![6-fluoroimidazo[1,5-a]pyridine](/CAS/20180531/GIF/1426421-17-1.gif)
1426421-17-1
10 suppliers
inquiry
Yield:1426421-17-1 517 mg
Reaction Conditions:
with trichlorophosphate in toluene at 100; for 4 h;
Steps:
32.3 Step 36-Fluoro-imidazo[l ,5-a]pyridine
Step 36-Fluoro-imidazo[l ,5-a]pyridineIn a round-bottomed flask, N-(5-fluoro-pyridin-2-ylmethyl)-formamide (612 mg, 3.97 mmol) was dissolved in toluene (16 ml) and phosphorus oxychloride (0.68 ml, 7.3 mmol) was added. The reaction mixture was stirred at 100°C in an oil bath for 4 h then cooled to 0°C and carefully quenched with ice. Aqueous 25% ammonium hydroxide ws added until pH=~9. The mixture was diluted with water and extracted with dichloromethane (3x). The organic layers were combined, dried over sodium sulfate, filtered and concentrated to give 517 mg (96%) of 6- fluoro-imidazo[l ,5-a]pyridine as a light brown oil. lU NMR (CDC13, 300 MHz): ? (ppm) 8.16 (s, 1H), 7.84 - 7.94 (m, 1H), 7.50 (s, 1H), 7.46 (dd, J=9.8, 5.3 Hz, 1H), 6.69 (ddd, J=9.8, 7.7, 2.1 Hz, 1H).
References:
WO2013/30138,2013,A1 Location in patent:Page/Page column 109
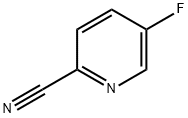
327056-62-2
256 suppliers
$5.00/1g
![6-fluoroimidazo[1,5-a]pyridine](/CAS/20180531/GIF/1426421-17-1.gif)
1426421-17-1
10 suppliers
inquiry
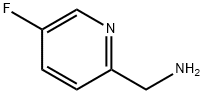
561297-96-9
80 suppliers
$47.00/100mg
![6-fluoroimidazo[1,5-a]pyridine](/CAS/20180531/GIF/1426421-17-1.gif)
1426421-17-1
10 suppliers
inquiry